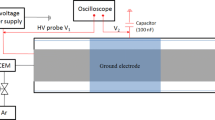
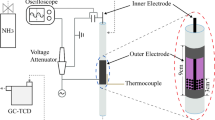
Abstract
The indirect synthesis of NH3 from N2 and H2O through plasma processing is proposed and demonstrated. NH3 is a promising hydrogen storage material because of its high hydrogen storage density. Mg3N2 is a key material for indirect NH3 synthesis, because the reaction of Mg3N2 with water easily generates NH3 at room temperature. In this paper, therefore, we focus on generating Mg3N2 by nitridation of MgO with nonthermal atmospheric-pressure dielectric barrier discharge (DBD) plasma in a N2 atmosphere. By intermittent DBD nitridation treatment while the reaction device was cooled in a water bath, a maximum Mg3N2 generation efficiency of 93 mg/kWh was estimated. Because NH3 is generated through a simple chemical reaction, our scheme does not cause NH3 decomposition by plasma, which is one of the greatest concerns associated with plasma synthesis. Contrary to the conventional NH3 generation process, which emits CO2 and requires high temperature and pressure, our scheme enables NH3 synthesis from N2 and H2O without CO2 emissions. This allows for an onsite small-scale NH3 synthesis system to be realized under mild conditions, which is necessary for a future low-carbon society.







Similar content being viewed by others
References
Wang W, Herreros JM, Tsolakis A, York APE (2013) Ammonia as hydrogen carrier for transportation; investigation of the ammonia exhaust gas fuel reforming. Int J Hydrog Energy 38:9907–9917
Patil BS, Wang Q, Hessel V, Lang J (2015) Plasma N2-fixation: 1900–2014. Catal Today 256:49–66
Yin KS, Venugopalan M (1983) Plasma chemical synthesis. I. Effect of electrode material on the synthesis of ammonia. Plasma Chem Plasma Process 3(3):343–350
Sugiyama K, Akazawa K, Oshima M, Miura H, Matsuda T, Nomura O (1986) Ammonia synthesis by means of plasma over MgO catalyst. Plasma Chem Plasma Process 6(2):179–193
Uyama H, Matsumoto O (1989) Synthesis of ammonia in high-frequency discharges. Plasma Chem Plasma Process 9(1):13–24
Uyama H, Matsumoto O (1989) Synthesis of ammonia in high-frequency discharges. II. Synthesis of ammonia in a microwave discharge under various conditions. Plasma Chem Plasma Process 9(3):421–432
Inoue Y, Kitano M, Kishida K, Abe H, Niwa Y, Sasase M, Fujita Y, Ishikawa H, Yokoyama T, Hara M, Hosono H (2016) Efficient and stable ammonia synthesis by self-organized flat Ru nanoparticles on calcium amide. ACS Catal 6:7577–7584
Bai M, Zhang Z, Bai X, Bai M, Ning W (2003) Plasma synthesis of ammonia with a microgap dielectric barrier discharge at ambient pressure. IEEE Trans Plasma Sci 31(6):1285–1291
Mizushima T, Matsumoto K, Ohkita H, Kakuta N (2007) Catalytic effects of metal-loaded membrane-like alumina tubes on ammonia synthesis in atmospheric pressure plasma by dielectric barrier discharge. Plasma Chem Plasma Process 27:1–11
Kim HH, Teramoto Y, Ogata A, Takagi H, Nanba T (2017) Atmospheric-pressure nonthermal plasma synthesis of ammonia over ruthenium catalysts. Plasma Process Polym 14:1–9
Veitch GE, Bridgwood KL, Ley SV (2008) Magnesium nitride as a convenient source of ammonia: preparation of primary amides. Organ Lett 10:3623–3625
Vissokov G, Grancharov L, Tsvetanov T (2003) On the plasma-chemical synthesis of nanopowders. Plasma Sci Technol 5(6):2039–2050
Kim D, Kim T, Park H, Park D (2011) Synthesis of nanocrystalline magnesium nitride (Mg3N2) powder using thermal plasma. Appl Surf Sci 257:5375–5379
Hanlon JM, Diaz LB, Balducci G, Stobbs BA, Bielewski M, Chung P, Maclaren L, Gregory DH (2015) Rapid surfactant-free synthesis of Mg(OH)2 nanoplates and pseudomorphic dehydration to MgO. CrystEngComm 17:5672–5679
Yabe T, Uchida S, Ikuta K, Yoshida K, Baasandash C, Mohamed MS, Sakurai Y, Ogata Y, Tuji M, Mori Y, Satoh Y, Ohkubo T, Murahara M, Ikesue A (2006) Demonstrated fossil-fuel-free energy cycle using magnesium and laser. Appl Phys Lett 89:2611070
Napartovich AP (2001) Overview of atmospheric pressure discharges producing nonthermal plasma. Plasma Polym 6:1–14
Gentile AC, Kushner MJ (1995) Reaction chemistry and optimization of plasma remediation of NxOy from gas streams. J Appl Phys 78(3):2074–2085
Acknowledgements
This work was supported by JSPS KAKENHI Grant Number 16H06790.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zen, S., Abe, T. & Teramoto, Y. Indirect Synthesis System for Ammonia from Nitrogen and Water Using Nonthermal Plasma Under Ambient Conditions. Plasma Chem Plasma Process 38, 347–354 (2018). https://doi.org/10.1007/s11090-017-9869-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11090-017-9869-8