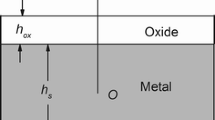
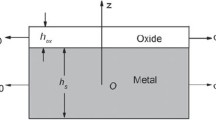
Abstract
The aim of this paper was to investigate the effect of external mechanical loads on the kinetics and process of high temperature oxidation of metals or alloys through a modeling approach. In this model, the complicated interplay among the external stress, growth strain and creep strain, the oxide stress and thickness was identified. The stress accumulation in the oxide and the variation of scale thickness along with time and the effect of oxidation behavior on the creep properties of underlying metal during oxidation process can be expected. Generally, the external tensile stress would promote the growth rate of oxidation layer. Introducing a small external stress may lead to obvious changes of magnitude and state of stress in oxidation layer. At a given external stress, the creep strain rate was not kept constant due to the oxidation layer growth and stress transfer between oxidation layer and underlying metal.













Similar content being viewed by others
References
F. N. Rhines and J. S. Wolf, Metallurgical Transactions A 1, 1970 (1701).
V. K. Tolpygo, J. R. Drygen and D. R. Clarke, Acta Materialia 46, 1998 (927).
V. K. Tolpygo and D. R. Clarke, Oxidation of Metals 49, 1998 (187).
H. E. Evans, International Materials Reviews 40, 1995 (1).
D. L. Douglass, Oxidation of Metals 1, 1969 (127).
D. R. Clarke, Acta Materialia 51, 2003 (1393).
S. Maharjan, X. C. Zhang, F. Z. Xuan, Z. D. Wang and S. T. Tu, Journal of Applied Physics 110, 2011 (063511).
S. Maharjan, X. C. Zhang and Z. D. Wang, Journal of Applied Physics 112, 2012 (033514).
S. Maharjan, X. C. Zhang and Z. D. Wang, Oxidation of Metals 77, 2012 (93).
J. L. Ruan, Y. M. Pei and D. N. Fang, Acta Mechanica 223, 2012 (2597).
J. L. Ruan, Y. M. Pei and D. N. Fang, Corrosion Science 66, 2013 (315).
R. K. Wilson and E. C. Aifantis, Acta Mechanica 45, 1982 (273).
D. J. Unger and E. C. Aifantis, Acta Mechanica 47, 1983 (117).
M. Schütze, Oxidation of Metals 44, 1995 (29).
G. Moulin, P. Arevalo and A. Salleo, Oxidation of Metals 45, 1996 (153).
C. Wagner, Journal of the Electrochemical Society 99, 1952 (369).
A. M. Limarga and D. S. Wilkinson, Acta Materialia 55, 2007 (189).
A. M. Limarga and D. S. Wilkinson, Acta Materialia 55, 2007 (251).
R. W. Neu and H. Sehitoglu, Metallurgical Transactions A 20, 1989 (1755).
R. W. Neu and H. Sehitoglu, Metallurgical Transactions A 20, 1989 (1769).
S. D. Antolovich, S. Liu and R. Baur, Metallurgical and Materials Transactions A 12, 1981 (473).
J. Reuchet and L. Remy, Metallurgical and Materials Transactions A 14, 1983 (141).
Y. Kraftmakher, Physics Reports 299, 1998 (79).
J. C. Li, Metallurgical Transactions A 9, 1978 (1353).
W. Przybilla and M. Schütze, Oxidation of Metals 58, 2002 (103).
J. L. Grosseau-Poussard, B. Panicaud and S. B. Afia, Computation Materials Science 71, 2013 (47).
K. Taneichi, T. Narushima, Y. Iguchi and C. Ouchi, Materials Transactions 47, 2006 (2540).
A. M. Huntz, G. Calvarin-Amiri, H. E. Evans and G. Cailletaud, Oxidation of Metals 57, 2002 (499).
G. Calvarin, A. M. Huntz and R. Molins, Materials at High Temperatures 17, 2000 (257).
E. S. Oh, J. R. Walton, D. C. Lagoudas and J. C. Slattery, Acta Mechanica 181, 2006 (231).
M. A. Kattis, Acta Mechanica 96, 1993 (37–46).
Y. H. Suo and S. P. Shen, Acta Mechanica 223, 2012 (29).
S. L. Hu and S. P. Shen, Acta Mechanica 224, 2013 (2895).
C. R. Hickenboth, J. S. Moore, S. R. White, N. R. Sottos and J. Baudry, Nature 446, 2007 (423).
L. C. Stephen, Nature 487, 2012 (176).
A. Mihalyi, R. J. Jaccodine and T. J. Delph, Applied Physics Letters 74, 1999 (1981).
A. Stesmans, D. Pierreux, R. J. Jaccodine and M. T. Lin, Applied Physics Letters 82, 2003 (3038).
H. E. Evans, D. J. Norfolk and T. Swan, Journal of the Electrochemical Society 125, 1978 (1180).
L. Gaillet, S. Benmedakhne, A. Laksimi and G. Moulin, Journal of Materials Science 38, 2003 (1479).
P. Hancock and J. R. Nicholls, Materials Science and Technology 4, 1988 (398).
R. Prescott and M. J. Graham, Oxidation of Metals 38, 1992 (233).
M. Schütze, Oxidation of Metals 24, 1985 (199).
C. Liu and A. M. Huntz, Materials Science and Engineering A 160, 1993 (113).
Acknowledgments
The authors are grateful for the support by National Natural Science Foundations of China (51175177, 51322510, 51371082). The author Xiancheng Zhang is also grateful for the support by Special Programs for Key Basic Research of Science and Technology Commission of Shanghai Municipality (13DJ1400202 and 12JC1403200).
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
If the dislocation in the boundary climbs rate during oxidation was dL/dt, then the growth strain rate can be expressed as [6]
where \( \theta \) represents the angle tilt boundary and d is the grain size, which are stress independent. Climb by a distance of \( \varDelta L \) requires the addition of n molecules of oxide per unit length, 1, of the dislocation line, i.e.,
where \( \varOmega_{ox} \) is the volume of a molecule of the oxide, and b is the Burgers vector of the edge dislocation in the grain boundary. In such a case, the dislocation climbing rate can be expressed as
Then, the lateral growth strain rate can be rewritten as
The climb of the dislocations is limited by the attachment of diffusing species to the dislocation cores, i.e., \( dn/1.dt \). The net rate of attachment to each dislocation core will be equal to the rate of the attachment by trapping minus the rate at which ions detach by the thermal excitation. If the traps are spaced an average distance a along the dislocation line, the net rate of attachment can be expressed as [6]
where A represents the cross-section for diffusing ion to attach to the dislocation core per unit length of dislocation, B is an effective diffusion jump frequency and E T is the trap energy. J v is the diffusion flux without any stresses in the oxide then the item \( J_{v} \cdot \exp \left( { - \sigma_{ox} \varDelta \varOmega /kT} \right) \) could be used to express the stress dependent diffusion flux.
This expression seems to conflict with that of Evans, i.e., \( J_{v} \cdot \exp \left( {\sigma_{ox} \varDelta \varOmega /kT} \right) \). However, Clarke [6] also mentioned that large compressive stress would decrease the diffusivity. Hence, \( \sigma_{ox} \) represents the stress level in Clarke’s expression while it represents the oxide stress in that of the Evans. Thus, their points coincide with each other. According to Clarke’s suggestion, the attachment rate was much large than the detach rate [6]. Thus, neglecting the second item of Eq. 34 and substituting it into Eq. 33, the lateral growth strain rate is proportional to the diffusion flux
It is well known that the scale growth rate was proportional to the diffusion flux, i.e.,
Hence, the lateral growth strain rate is proportional to the scaling rate and this relationship can be expressed as
where the lateral growth constant, D ox , is determined by some stress independent parameters, including \( \theta \), d, \( \varOmega_{ox} \), A, a and b et al.
Rights and permissions
About this article
Cite this article
Zhang, Y., Zhang, X., Tu, ST. et al. Analytical Modeling on Stress Assisted Oxidation and its Effect on Creep Response of Metals. Oxid Met 82, 311–330 (2014). https://doi.org/10.1007/s11085-014-9493-4
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11085-014-9493-4