Abstract
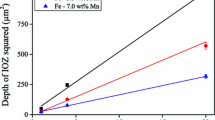
The transition from internal to external oxidation of Mn-steels depends on temperature, oxygen partial pressure or dew point and alloy composition. A description for this transition includes, besides the diffusion coefficients of Mn and oxygen, also on the critical volume fraction oxide precipitates. This volume fraction for Mn-steel alloys has been determined experimentally and equals 0.2, whereas usually a value of 0.3 is adopted. A low critical volume fraction oxide implies that the alloy is more prone to external oxidation. Oxidation experiments with 1.7, 3.5 and 7.0 wt% Mn-steel alloys at 950 °C, i.e. in the austenitic region, at different oxygen partial pressure or dew points in the range of −45 to +10 °C showed excellent agreement with the oxidation mode predicted using the data obtained for the diffusivities and critical volume fraction oxide.







Similar content being viewed by others
Notes
The grading system for abrasives is FEPA standard, Federation of European Products of Abrasives.
References
C. Wagner, Zeitschrift für Elektrochemie 63, 772 (1959).
R. A. Rapp, Acta Metallurgica 9, 730 (1961).
F. Gesmundo, Y. Niu, F. Viani and F. C. Rizzo, Oxidation of Metals 46, 441 (1996).
Y. Niu, X. J. Zhang, Y. Wu and F. Gesmundo, Corrosion Science 48, 4020 (2006).
R. Khondker, A. Mertens and J. R. McDermid, Materials Science and Engineering A 463, 157 (2007).
F. S. Pettit, Transactions of the Metallurgical Society of AIME 239, 1296 (1967).
J. A. Nesbitt, Journal of the Electrochemical Society 136, 1511 (1989).
F. Gesmundo and Y. Niu, Oxidation of Metals 50, 1 (1998).
G. M. Song, W. G. Sloof, T. Vystavel and J Th M De Hosson, Materials Science Forum 539–543, 1104 (2007).
M. Shibata, JEOL News 39, 28 (2004).
J. T. Armstrong, Quantitative elemental analysis of individual microparticles with electron beam instruments, in Electron Probe Quantitation, K. F. J. Heinrich and D. E. Newbury (Editors), (Plenum Press, 1991), p. 261.
ImageJ, image processing and analysis in Java, available from: http://rsbweb.nih.gov/ij/.
A. S. M. Handbook, Metallography and Microstructures, vol. 9, (ASM International Materials Park, Newbury, 1992), pp. 124–133.
W. M. Haynes (ed.), CRC Handbook of Chemistry and Physics, 93rd ed. (Internet version 2013), (CRC Press/Taylor and Francis, Boca Raton, 2012–2013), p. 4.
R. Janakiraman, G. H. Meier and F. S. Pettit, Metallurgical and Materials Transactions A 30, 2905 (1999).
M. C. Maris-Sida, G. H. Meier and F. S. Pettit, Metallurgical and Materials Transactions A 34, 2609 (2003).
C. Wagner, Journal of the Electrochemical Society 99, 369 (1952).
J. Crank, The Mathematics of Diffusion, (Clarendon Press, Oxford, 1975).
R. A. Rapp, Corrosion 21, 382 (1962).
D. R. Gaskell, Introduction to the Thermodynamics of Materials, 4th ed, (Taylor & Francis, New York, 2003), p. 547.
F. Gesmundo and F. Viani, Oxidation of Metals 25, 269 (1986).
M. Kahlweit, Zeitschrift für Physikalische Chemie Neue Folge 32, 1 (1962).
Thermo-Calc Software, available from: http://www.thermocalc.com/.
J. Takada, S. Yamamoto, S. Kikuchi and M. Adachi, Metallurgical Transactions A 17, 221 (1986).
J. H. Swisher and E. T. Turkdogan, Transactions of the Metallurgical Society of AIME 239, 426 (1967).
K. Nohara and K. Hirano, Transactions of the Iron and Steel Institute of Japan Metals Suppl 11, 1267 (1971).
H. Oikawa, Tetsu-To-Hagane 68, 1489 (1982).
D. Huin, P. Flauder and J.-B. Leblond, Oxidation of Metals 64, 131 (2005).
I. Barin, Thermochemical Data of Pure Substances, 3rd ed, (VCH, Weinheim, 1995), p. 796.
Acknowledgments
This research was carried out under project number MC7.07295a in the framework of the Research Program of the Materials innovation institute (M2i, www.m2i.nl). Financial support from International Zinc Association (IZA, www.zinc.org) is gratefully acknowledged. The authors are indebted to Dr. W. Melfo, Dr. H. Bolt and Dr. M. Zuiderwijk of Tata Steel (IJmuiden, The Netherlands) for valuable discussions and providing the Mn steel alloys. The authors are also grateful to Ing. J. C. Brouwer for all his technical support and assistance with the sample preparation.
Author information
Authors and Affiliations
Corresponding author
Appendices
Appendix 1
The relation between dew point (DP) and partial pressure of water vapor follows from [28]:
where the units of partial pressure of water vapor (\( p_{{H_{2} O}} \)) and dew point (DP) are in atmosphere and degree Celsius, respectively. Next, the corresponding partial pressure of oxygen can be calculated with the Gibbs free energy of formation (\( \Updelta G_{{H_{2} O}}^{0} \)) of water vapor [29]:
in which T is the absolute temperature in Kelvin.
Appendix 2
For the oxygen solubility in Fe it holds that [25]:
Here \( N_{O}^{(s)} \)denotes the surface concentration of oxygen in mole fraction. \( p_{{H_{2} O}} \) and \( p_{{H_{2} }} \) are the partial pressure of water vapor and hydrogen in atm., respectively, and T is the absolute temperature in Kelvin.
Appendix 3
In order to obtain the spatial size distribution of internal oxide precipitates from the area observed in the cross-sections, the Saltykov area method [13] was adopted. This method is based on the relative section areas of the precipitates, i.e. A/A max , where A max is the area of the largest precipitate. When assuming that the precipitates are spherical, then the area A can be related to its diameter D. Next, a series of size groups j is obtained by taking the logarithm of the ratio pertaining to the upper boundary of the subsequent size groups equal to 10−1, i.e. \( \log \left( {\frac{{D_{j + 1} }}{{D_{j} }}} \right) = 10^{ - 1} \)
Here, 12 size groups were considered. Next, the number of internal oxide particles divided by the total test area (IOZ) for all of the size groups \( (N_{A} )_{i} \) was counted (using ImageJ software [12]). These values are substituted in the so-called working equation, which reads [13]:
where \( (N_{v} )_{j} \) represents the number of particles per unit volume in the j th class interval, where \( j \in \left\{ {1,12} \right\} \). The largest particle size corresponds to j = 1. For each selected value of j, i is set equal to j, which determines the number of terms used inside the brackets. \( D_{j} \) is the maximum diameter of each size group concerned. The total number of particles per unit volume, \( N_{V} \), equal the sum of the \( (N_{v} )_{j} \) values, hence:
The mean diameter of the precipitates is obtained from [13], i.e.:
Rights and permissions
About this article
Cite this article
Lashgari, V.A., Kwakernaak, C. & Sloof, W.G. Transition from Internal to External Oxidation of Mn Steel Alloys. Oxid Met 81, 435–451 (2014). https://doi.org/10.1007/s11085-013-9456-1
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11085-013-9456-1