Abstract
Piezoelectric Crystal Microbalances (PCM’s) are widely used to study the chemical processes involving volatile compounds in any environment, such as condensation process. Since PCM’s are miniaturized sensor, they are very suitable for planetary in situ missions, where can be used to detect and to measure the mass amount of astrobiologically significant compounds, such as water and organics. This work focuses on the realization and testing of a new experimental setup, able to characterize volatiles which can be found in a planetary environment. In particular the enthalpy of sublimation of some dicarboxylic acids has been measured. The importance of dicarboxylic acids in planetology and astrobiology is due to the fact that they have been detected in carbonaceous chondritic material (e.g. Murchinson), among the most pristine material present in our Solar System. In this work, a sample of acid was heated in an effusion cell up to its sublimation. For a set of temperatures (from 30 °C to 75 °C), the deposition rate on the PCM surface has been measured. From these measurements, it has been possible to infer the enthalpy of sublimation of Adipic acid, i.e. ΔH = 141.6 ± 0.8 kJ/mol and Succinic acid, i.e. ΔH = 113.3 ± 1.3 kJ/mol. This technique has so demonstrated to be a good choice to recognise a single compound or a mixture (with an analysis upstream) even if some improvements concerning the thermal stabilization of the system will be implemented in order to enhance the results’ accuracy. The experiment has been performed in support of the VISTA (Volatile In Situ Thermogravimetry Analyzer) project, which is included in the scientific payload of the ESA MarcoPolo-R mission study.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dicarboxylic acids are organic compound with a general chemical formula written as HOOC(CH 2) n − 2 COOH where n is the number of carbon atoms. The dicarboxylic acid contain two carboxylic acid functional groups and are used to prepare copolymers and polyamides and polyesters. This kind of acid is present with various concentrations in different terrestrial environments (e.g. marine, rural, urban) (Kawamura et al. 2005; Yu and Fraser 2004; Limbeck et al. 2001) and their presence in atmosphere is likely due to volatile organic compound oxidation .
The Dicarboxylic acids(with a carbon chains fromC 2 to C 12) are emitted into terrestrial atmosphere in both gaseous and particulate form from photo-oxidation of biogenic and anthropogenic sources such as fossil fuel burning, forest fires, meat-cooking and cigarette smoke (Bilde et al. 2003).
Dicarboxylic acids have been found also in carbonaceous chondrites (Andersen and Haack 2005; Briscoe and Moore 1993), meteorites associated to the primitive asteroids; and hence could give information about chemical and mineralogical composition of the early Solar System.
Chemical analyses on carbonaceous chondrites revealed a variety of organic compounds, including amino acids, aliphatic and aromatic hydrocarbons, mono-Carboxylic acids and some Dicarboxylic acids, such as Succinic acid and Adipic acid in small quantities (5.9 μg/g and 0.7 μg/g, respectively). In this scenario, the presence of the Succinic Acid is consistent with a synthesis from hydrogen cyanide and ammonia. In particular the Succinic acid/β-Alanine (Amino acid) ratio makes it possible to estimate the relative period of synthesis of organic compounds on the Murchinson meteorite parent body (Peltzer et al. 1984).
Piezoelectric Crystal Microbalances can be applied in planetary in situ mission in order to detect the possible presence of water and organics (linked to the habitability of the planetary body) in dust or aerosol in different environments. The Piezoelectric Crystal Microbalance (PCM) oscillation frequency goes as the inverse of the mass sample deposited on it, as stated by the Sauerbrey equation (Sauerbrey 1959). If it is possible to increase or decrease the PCM temperature, the sublimation or condensation of volatile components can be allowed (Fig. 1). The amount of volatile components will be given by the mass variation during the sublimation/condensation process whereas its composition can be inferred by the sublimation/condensation temperature.
However, the identification of the sublimated/condensed volatiles can be a tricky task due to similar desorption temperatures of some compounds. In particular, if there are present impurities or materials mixture the detection is more complicated due to the variation of the physical properties (i.e. sublimation temperature, enthalpy, Gibbs free energy etc.) of the substance. In this case a chemical analysis can help to reveal the material composition. Considering a pure substance (our case), for a good evaluation the enthalpy results should be within 10 % (section 4) of the expected value. In this manner, it is possible to use this approach for recognise the volatiles by means the thermodynamic properties.
Then, the compound needs to be characterised by another thermal properties, such as the enthalpy of sublimation/evaporation (ΔH), which characterizes a compound during a chemical-physical process.
Many experimental methods allow to measure evaporation rates and to retrieve the enthalpy of sublimation (by means of application of Clausius-Clapeyron and Langmuir equations) of dicarboxylic and monocarboxylic acids, e.g. Thermal Desorption Particle Beam Mass Spectrometry Method (TPTD, Chattopadhyay and Ziemann 2005), where at pressure of 6 × 10−8 mbar and with a sample thermal desorption it is possible to measure the evaporation rates (monitored in real time using a mass spectrometer); Knudsen Effusion Mass-loss (da Silva et al. 2001), based on simultaneous operations of three Knudsen cell at pressure of 5 × 10−7mbar; Knudsen Effusion Mass Spectrometry (Booth et al. 2009), a method for estimate the vapor pressure by quadrupole mass spectrometer in a vacuum chamber (10−6mbar) and controlling the Knudsen effusion cell temperature; Tandem Differential Mobility Analyzer technique (TDMA, Bilde et al. 2003; Mɵnster, et al. 2004) which allows to measure the vapor pressure of organic particles at ambient temperature.
In this work, we instead used PCM’s to measure the enthalpy of sublimation for two Dicarboxylic acids, i.e. Adipic and Succinic acid. Cooling the PCM at −72 °C it was possible to use the microbalance as mass attractor for the acid molecules (Fig. 1, Left). Then, during the heating of an effusion cell containing pure substance, the sublimation process was monitored (facilitated by the low pressure of the environment) by measuring deposition rates on the PCM. Finally, the enthalpy of sublimation was measured between two temperature step (ΔT = 5 °C), from 30 °C to 75 °C.
By using Clausius-Clapeyron equation (fitting method) or Van’t Hoff relation (to estimate the enthalpy variation between two nearby temperature) it is possible to estimate the enthalpy for each temperature intervals.
Scientific Background
The MarcoPolo-R Mission and the VISTA Instrument
MarcoPolo-R (M3-ESA mission study) is a sample return mission (Barucci 2011) which aims to bring to Earth samples (100 g) of a primitive Near-Earth Asteroid (NEA), i.e. 2008EV5. The returned samples will undergo to laboratory tests, which will give insights aboutthe origin of planetary materials, the early Solar System formation processes and the organic and volatile substances in a primitive asteroid, focusing on the connection between these materials and biogenic molecules. Andersen and Haack (2005) and Briscoe and Moore (1993) proposed that complex organic molecules (Amino acids, Nucleobases and Carboxylic acids) contained in carbonaceous chondrites have been able to triggering the prebiotic synthesis of biochemical compounds in the early Earth. The VISTA (Volatile In Situ Thermogravimetry Analyser) μ-Thermogravimeter (Fig. 2) is included in the MarcoPolo-R scientific package (Palomba et al. 2012) and is based on PCM’s. Among its science objectives, it plans to measure water and organic content in the asteroidal regolith.
VISTA breadboard. Left: the PCM sensor with a built in thermistor and built in heater (around the gold electrode). The heater provided the mass variation on the crystal surface whereas the thermistor acts to temperature control. Right the proximity electronic (PE),which includes a reference oscillator and a control for heater and thermistor
Thermodynamic Relations
The enthalpy of phase transition is given by the difference between energy of the reactants and energy of the products. In a phase transition as sublimation process the enthalpy consist in the internal energy of the system plus the product of pressure (p) and volume (V) of the system. Considering a ideal gas, this thermodynamic quantity is stated as:
The Clausius – Clapeyron relation is generally used to characterize a phase transition and to detect the vapour pressure variation with the temperature
being ΔH, the specific latent heat of the process (sublimation, vaporization, or fusion), p the vapour pressure and ΔV the difference between the volume of the gas formed and the volume of solid or liquid (sublimation/vaporization phase). Assuming the ΔV ~ Vgas = RT/p the equation can be expressed in differential form
Therefore, introducing the Langmuir equation for evaporation in vacuum, it is possible to determine the pressure of the gas by means the experimentally measured deposition rate or dm/dt (the mass loss rate per unit area)
where is p the vapour pressure, M the molecular weight, R the gas constant, T the absolute temperature and α the vaporization coefficient (assumed to be 1 in vacuum environment) (Price 2001). Using the equation (4) in equation (3), after the integration it is possible to obtain the enthalpy of sublimation from the slope of the curve: (dm/dt)T1/2 versus 1/T
Moreover, in order to monitor the enthalpy variation step by step in the temperature interval (this work), the Van’t Hoff relation can be used. Measuring at two different temperature the mass loss rate by means the PCM frequency the enthalpy was monitored. The Van’t Hoff relation is typically used to explore the change in state function in a thermodynamic system as the change in enthalpy, Gibbs energy, and entropy, or amount of disorder in a chemical reaction.
This approach allows to link a term which includes a mass variation \( \ln \Big(\frac{dm}{dt}{T}^{1/2} \)) of a chemical process to the temperature, T −1. Then, using two temperatures (or the whole temperature range, if the fitting method is used) the enthalpy variation of the physical-chemical process can be monitored.
Considered Materials and Experimental Setup
Dicarboxylic Acids
The considered Dicarboxylic acid samples consist in small grains of white crystalline form of Adipic acid and Succinic acid, respectively, with a purity degree of 99 %. In this work we have used the samples with n = 4 (Succinic acid) and n = 6 (Adipic acid). The Adipic acid has the chemical formula (CH 2)4(COOH)2 and it is usually produced every year in the industrial field as monomer for the production of nylon and is also produced by the oxidation in the terrestrial atmosphere, linked to emissions of N 2 O, a potent greenhouse gas and cause of stratospheric ozone depletion. The Succinic acid with a chemical formula HOOC − (CH 2)2 − COOHis an odorless solid. In the biochemistry field, the Succinic acid plays an important role in the citric acid cycle (Krebs cycle) and in the industrial field it is a precursor to some specialized polyesters. As seen in previous studies (i.e. Murchinson meteorite, Peltzer et al. 1984), thermochemical analyses are important for detection of these substances in different planetary bodies or in the terrestrial atmosphere.
The setup operates in the temperature range from 30 °C up to 110 °C, which includes the sublimation point of the two considered acids (i.e. about 80 °C for the Adipic and 60 °C for the Succinic).
Some structural and thermodynamic characteristics of adipic and succinic acid are shown in Table 1(Afeedy, H.Y., NIST 1998).
Experimental Setup
In order to monitor the enthalpy of sublimation variation step by step we measure the deposition rates on the PCM, by measuring the frequency change (i.e. variation of deposited mass) of the microbalance.
The PCM, with a resonant oscillation frequency of 10 MHz, is cooled by a liquid nitrogen serpentine whereas the acid sample is placed in effusion cell (a small case, with an evaporation hole wide 6 mm and deep 10 mm). The sample is then heated by a resistance up to its sublimation.
By heating the sample and measuring the corresponding deposition rates, the enthalpies of sublimation of Adipic and Succinic acid and the relative deposition curve from 30 °C to 75 °C may be obtained.
In our experiment, the aim of work was monitor the variation of the enthalpy of sublimation across the temperature interval. Assuming the rate constant k as the deposition rate on the PCM (dm/dt: the molecules that perform the phase transition solid-gas) and multiplied for the term T1/2, the Van’t Hoff relation (Benson 1968) was applied. By using the Eq. (5) and by measuring two different rate constants k 1 and k 2 at the respective temperatures T 1 and T 2, the enthalpy of sublimation can be obtained:
Therefore, by measuring the constant rate at each type of reaction (sublimation, vaporization, or fusion ) we are able to infer this termochemistry variable.
Then, the acid sample (13.0 ± 0.5 mg for Adipic acid and 13.0 ± 0.5 mg for Succinic acid) are positioned in the effusion cell and subjected to the thermal cycle operated by a Proportional-Integral-Derivative controller (PID). We applied a stepwise temperature increase of 5 °C (in order to discern two molecule fluxes at different temperatures), with the sample maintained at each temperature for 30 min in order to allow the stabilization of the relative deposition rates.
The temperatures are monitored with platinum sensors (PT100) whose resistance changes linearly with the temperature.
The PCM is connected to a copper plate (in thermal contact with a coil containing liquid nitrogen) and enclosed in a metal casing (drilled in the center) as to favour the cooling for irradiation. With this method the PCM is cooled down to −72 °C in order to help the condensation of the acid molecules released for sublimation process.
The PCM and the effusion cell with the sample are placed in a sublimation chamber (of insulating material) for minimise thermal dispersion and avoid the loss of the molecules into the surrounding environment(Fig. 3,left).
Left: the sublimation chamber containing the effusion cell, the sample and the PCM. Right: schematic representation of the setup. The sample is contained in the effusion cell and sublimated by means of a resistance which heats the cell. The condensation of sublimated molecules on the PCM is allowed by cooling the microbalance, in contact with a coil containing liquid nitrogen. In order to avoid heat and flux dispersion, the effusion cell and the PCM are isolated from the rest of the vacuum chamber and are contained in an insulating sublimation chamber (covered by Teflon)
Furthermore, in order to avoid the dispersion of the molecules flow on the metal casing, the PCM and the sample are directly positioned at only distance 2 cm.
The effusion cell is connected to a resistance of 25 W that heats the compound up to its sublimation. On the other side, the PCM is cooled down to −72 ± 1 °C in order to favour the condensation of the acid molecules released for sublimation on the PCM. The whole setup is placed inside a vacuum chamber (internal pressure of 10−6 mbar) in order to simulate the space environments (Fig. 3, right).
Hence, the deposition of the acid molecules cause PCM frequency changes. This frequency variation is then converted in variation of the mass deposited on the crystal surface. At each temperature step it is possible to measure the acid deposition rate in Hz/s.
By measuring the acid deposition rates on the PCM, k 1 and k 2 (considering also the term T1/2), at two different sample temperatures, T 1and T 2, it is possible to infer the enthalpy of sublimation of the compound (Equation 6). The considered temperatures span from 30 °C to 75 °C for the Adipic acid and from 30 °C to 55 °C for the Succinic acid. The different ranges considered for the two acids are due to the fact that the Succinic acid has a lower sublimation temperature and the application of the Van’t Hoff relation does not give reliable results beyond the sublimation point (≥60 °C), as we will see in the next session. In the analysis, the sublimation point is the threshold where the sample sublimes in steadily mode.
The enthalpies of sublimation obtained by the measured deposition rates were compared to the literature values (Albyn 2004; Booth et al. 2009; Chattopadhyay and Ziemann 2005; da Silva et al. 2001; Bilde et al. 2003; Mɵnster, et al. 2004),
Results and Discussion
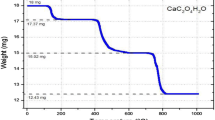
The analysis showed an increase of the deposition rates of the acid on the PCM at increasing temperature, corresponding to a PCM frequency decrease. For the Adipic acid we observed a frequency variation well higher than that observed for the Succinic acid: in the first case the total frequency decrease was 27 kHz from 30 °C to 75 °C (Fig. 4), whereas in the second case it was 11 kHz from 30 °C to 55 °C (Fig. 5).
The efficiency of the condensation process is larger when the difference between the PCM surface and the effusion cell temperatures increases and when the molecules flux are focused directly on the PCM crystal (Dirri et al. 2012).
In order to monitor the enthalpy variation over the whole temperature range considered, a good choice for T 2 could be a temperatures close to the sublimation point (i.e. from 60 °C to 75 °C for Adipic acid and from 50 °C to 60 °C for Succinic acid). Otherwise, at temperatures T 1 and T 2 too far or too close to the sublimation point, it is difficult to measure the enthalpy of sublimation with a good accuracy, because it is difficult to estimate the enthalpy in a narrow temperature range (5 °C in the worst case). Considering the different methods (KEMS, TDMA etc.) and the problem linked to the choice of the temperature interval, the enthalpies could not be in agreement with the literature values (see Table 2). Taking into account the available setup a range for acceptable error of ≤5 percent might be a sufficient threshold to provide a good accuracy for these measurements. In some acquisition data, the systematic error plays an important role on the measurement process, in particular on the temperature evaluation at each set point. The direct effect is a larger error on the final enthalpy of sublimation (±15 %, Table 2 and 3).
The Succinic acid shows an increase in the deposition rate up to the sublimation temperature (between the 55 °C and 60 °C). Beyond the sublimation point (i.e. about 60 °C), it is not possible to observe a monotonous trend of the frequency rate with the sample temperature, due to the constant sublimation flux of the substance (Table 3 and Fig. 5). The obtained rate in Hz/s has been multiplied for the QCM sensitivity (which is 4.4 ng/cm 2) and converted in (gr/cm 2 × s). Subsequently, dividing by the substance molecular weight (i.e. 146.14 and 118.09 for the Adipic and Succinic acid, respectively) the deposition rate has been converted in (mol/cm 2 × s). The enthalpy of sublimation and its error have been retrieved in kJ/mol, together with Δ, i.e. the percentage difference of calculated enthalpy compared to literature value (Chattopadhyay and Ziemann 2005). The uncertainty on temperature and deposition rate has been calculated as standard deviation of the mean, due to the Gaussian distribution of the measured values. When the data did not reproduce a normal distribution, the standard deviation was assumed as uncertainty on the measurement. This assumption leads to errors up to 15 % (underline values in the Table 2 and 3). On the contrary, when the standard deviation of the mean is assumed as uncertainty, the error is ≤4%.
Even if the temperature steps are set every 5 °C in our experiments, the results are in agreement with the literature (within ±10 %) as long as T 1 is far away from the sublimation point, i.e. at least 10 °C lower (Table 3). Besides, when T 1 approaches T 2, it is possible that the results obtained are in disagreement with the literature and the errors on the enthalpy of sublimation are ≥10 kJ/mol (Adipic and Succinic acid, due the narrow ΔT of two consecutive flux).
In Table 4, it is possible to observe a comparison with enthalpies calculated in this work and the enthalpy literature values obtained with other techniques (TPTD, KEMS, TDMA).
The obtained enthalpy of the Succinic acid, is in agreement within ±10 %, with the results reported by Booth et al. (2009); Da Silva et al. (2001) and Chattopadhyay and Ziemann (2005) while the Adipic acid result is in agreement (within ±10 %) with Bilde et al. (2003); Mɵnster, et al. (2004); Chattopadhyay and Ziemann (2005) and Albyn (2004) results (Table 4), which applied a procedure similar to that adopted in this work. In the Albyn (2004) experiment the PCM temperature was set to −62 °C, with the acid sample positioned in a effusion cell placed in a vacuum chamber at pressure of 10−7mbar.
The main observed differences are probably due to the temperature and pressure used in the various experiments that led to different evaporation rates or vapour pressures at each temperature step.
Conclusions
We developed a setup to calculate the enthalpy of sublimation of low volatile organics, based on Piezoelectric Crystal Microbalances. These devices have the strong advantage to be light and hence suitable for planetary in-situ measurements.
This setup has been tested by measuring the enthalpy of two Dicarboxylic acids, i.e. the Adipic and the Succinic acid, and comparing the retrieved results with those obtained with other techniques. The samples have been analyzed in a controlled environment (vacuum chamber at 10−6 mbar): they have been heated in an effusion cell in order to allow the molecule condensation on a cooled PCM (held at −72 °C). The results of the calculated enthalpies are in agreement within 10 % with the literature values (except for Booth et al. 2009, Adipic acid sample). For some results (see Table 2-3) the larger uncertainty is due to the systematic error present in the evaluation of the temperature and hence in the molecules flux. Therefore, a setup improvement is necessary in order to indentify a single volatile compound by its enthalpy. In particular, focusing the molecules flux on the QCM electrode (keeping out the metal case by the molecules field of view) and modifying the temperature control system (that increases the uncertainty up to 15 % on each measurement).
In fact, excluding the results obtained without an accurate temperature control (~1 °C) the enthalpy values show a good accuracy, within 3.5 % for the Adipic acid and 4 % for the Succinic acid.
Therefore, the exposed results show the good performance of PCM to measure the enthalpy of sublimation of these known species, present in small quantities in the chondrites carbonaceous, at different temperature in vacuum (which best simulated the space conditions).
It is planned to apply this technique to other acids and compounds of planetary interest, in particular meteorite samples, in order to characterize the organic compounds present within this material. This will imply a modification of the setup and also extend the working temperature range in order to analyzed compounds with different sublimation temperature.
References
Afeedy HY et al (1998) “Phase change Data”; in NIST Chemistry WebBook, NIST (National Institute of Standards and Technology) Standard Reference Database No. 69 (online),URL: http://webbook.nist.gov
Albyn KC (2004) Quartz Crystal Microbalance Operation and In Situ Calibration, Alabama, NASA/TM-213550. Memorandum, Technical
Andersen CA, Haack H (2005) Carbonaceous chondrites: tracers of the prebiotic chemical evolution of the solar system. Int J Astrobiol 4(1):13–17
Barucci, M.A. et al. (2011), MarcoPolo-R near earth asteroid sample return mission, Exp Astron, 33, 2–3, 645–684
Benson SW (1968) Thermochemical Kinetics, 2nd edn. John Wiley & Sons, New York, NY, p. 1017
Bilde M., Svenningsson B., Mɵnster J., and Rosenɵrn T. (2003), Even-odd alternation of evaporation rates and vapor pressures of C3-C9 dicarboxylic acid, aerosols Environ Sci Technol 37, 1371–1378
Booth AM, Markus T, McFiggans G, Percival CJ, Mcgillen MR, Topping DO (2009) Design and construction of a simple Knudsen Effusion Mass Spectrometer (KEMS) system for vapour pressure measurements of low volatility organics. Atmos Meas Tech 2:355–361
Briscoe JF, Moore CB (1993) Determination of formic and acetic acid in chondritic meteorites. Meteoritics 28(3):330
Chattopadhyay S, Ziemann PJ (2005) Vapor pressures of substituted and unsubstituted monocarboxylic and dicarboxylic acids measured using an improved thermal desorption particle beam mass spectrometry method. Aerosol Sci Technol 39(11):1085–1100
da Silva RMAV, Monte Manuel J. S, Ribeiro JR (2001) Thermodynamic study on the sublimation of succinic acid and of methyl- anddimethyl-substituted succinic and glutaric acids. J Chem Thermodynamics 33:23–311
Dirri F, Palomba E, Longobardo A, Zampetti E, Biondi D, Boccaccini A, Pantalei S, Zinzi A (2012) Measuring enthalpy of sublimation of volatiles by means of micro-thermogravimetry for the study of water and organic in planetary environments, Proceedings of XI National Science Planetary Congress, 04–08 February. Bormio, Italy
Kawamura K, Imai Y, Barrie LA (2005) Photochemical Productionand Loss of Organic Acids in High Arctic Aerosols DuringLong-Range Transport and Polar Sunrise Ozone DepletionEvents. Atmos Environ 39:599–614
Limbeck A, Puxbaum H, Otter L, Scholes MC (2001) SemivolatileBehavior of Dicarboxylic Acids and Other Polar Organic Species at a RuralBackground Site (Nylsvley, RSA). Atmos Environ 35:1853–1862
Mɵnster, J., Rosenorn T., Svenningsson B., Bilde M., (2004), Evaporation of methyl- and dimethyl-substituted malonic, succinic, glutaric and adipic acid particles at ambient temperatures, Elsevier, J Aerosol Sci 35, 1453–1465
Palomba E, Longobardo A, Dirri F, Zinzi A, Biondi D, Zampetti E, Pantalei S, Saggin B, Bearzotti A, Macagnano A, Brucato JR, Keheyan Y (2012) VISTA: a thermogravimeter for the MarcoPolo-R mission, 39th COSPAR, Scientific assembly, held 14–22 June 2012. Mysore, India
Peltzer ET, Bada JL, Schlesinger G, andMillerS L (1984) The chemical conditions on the parent body of the Murchinson meteorite: some conclusions based on amino, Hydroxy and dicarboxylic. Adv Space Res 4(12):69–74
Price D. (2001), Vapor Pressure determination by Thermogravimetry, Thermoch. Acta 367–368, 253–262, Elsevier
Sauerbrey G (1959) Verwendung von Schwingquarzen zur Wägung dünner Schichten und zur Mikrowägung. Z Phys 155:206–222
Yu Z, Fraser MP (2004) Polar organic compounds measured in fine particulate matter during TexAQS 2000. Atmos Environ 38:3253–3261
Acknowledgments
The authors thank Angelo Boccaccini (IAPS-INAF), David Biondi (IAPS-INAF) and Angelo Zinzi (ASDC-OAR) to their technical support and the Institute of Translational Pharmacology (ITF-CNR, Italy)for the substances used in the experiments.
Author information
Authors and Affiliations
Corresponding author
Additional information
This paper is part of the Special Collection of Papers from EANA 2013: The 13th European Workshop on Astrobiology, 22–25 July 2013, Szczecin, Poland (Franco Ferrari and Ewa Szuszkiewicz Guest Editors).
Rights and permissions
About this article
Cite this article
Dirri, F., Palomba, E., Longobardo, A. et al. Measuring Enthalpy of Sublimation of Volatiles by Means of Piezoelectric Crystal Microbalances. Orig Life Evol Biosph 47, 533–544 (2017). https://doi.org/10.1007/s11084-016-9517-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11084-016-9517-y