Abstract
S-Isovaline (S-Iva: 6.7 mmol) and D,L-glutamic acid (Glu: 2 mmol) were dissolved in 10 ml of hot water, and the resulting solution was divided in 5 vessels. After recrystallization, the crystals were collected from each vessel, and the enantiomeric excess (ee) of Glu was determined with chemical derivatization using 1-fluoro-2,4-dinitrophenyl- 5-L-leucinamide followed by high-performance liquid chromatography. Ten crystallizations provided all D-rich Glu with ee values of 2.69 % ± 0.81 % (mean ± standard deviation), and those using R-Iva provided all L-rich Glu with ee values of 6.24 % ± 2.20 %. Five recrystallizations of D,L-Glu alone provided ee values of 0.474 % ± 0.33 %. The differences among these three ee values were statistically significant, showing that S-Iva, which was present in meteorites caused a significant induction of ee in this physiological amino acid. This is the first outcome that S-Iva induced ee changes in a physiological amino acid. S-Iva did not induce any ee changes in D,L-asparagine, leucine, valine, methionine, phenylalanine, tryptophan, glutamine, tyrosine, aspartic acid, or histidine under similar recrystallizations.
Similar content being viewed by others
Introduction
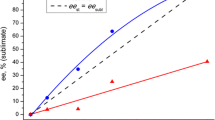
Many hypotheses on the origin of the homochirality of amino acids on prebiotic earth have been proposed (Avalos et al 2004; Kojo 2010), but theories with experimental evidence are limited. As a symbolic study, Breslow and Levine (2006) showed that solutions with 1 % enantiomeric excess (ee) of D- or L-phenylalanine (Phe) were amplified to 90 % ee by two successive evaporations to precipitate the racemate. This study shows the difficulty of inducing the tiny 1 % ee from the racemate, which is required for chiral amplification (Soai et al 1995).
Furthermore, there is an important problem concerning the generation of different L-amino acids. Even if L-Phe is generated by a specific mechanism, this mechanism is not always applicable for generating other L-amino acids. Therefore, in theory, 19 mechanisms would be required for forming 19 L-amino acids.
We previously reported that racemic D,L-asparagine (Asn) induced ee by simple recrystallization from water (Kojo and Tanaka 2001). In addition, D,L-Asn caused the spontaneous asymmetric resolution of co-existing D,L-amino acids, such as Phe, tryptophan (Trp), arginine (Arg), aspartic acid (Asp), histidine (His), leucine (Leu), methionine (Met), serine (Ser), tyrosine (Tyr), and valine (Val), by recrystallization. The ee values of the co-existing amino acids in the resulting crystals correlated linearly with that of Asn: namely, L-Asn crystallized preferentially with other L-amino acids, and D-Asn crystallized preferentially with other D-amino acids (Kojo and Tanaka 2001; Kojo et al 2004). These observations indicated that recrystallization was a mechanism that led to a set of L- or D-amino acids, and the 19 independent mechanisms for each amino acid were not necessary. However it was incidental whether the enrichment occurred either in L- or in D-Asn.
To determine the conditions that selectively generate L-amino acids, we examined whether isovaline (Iva), which was contained in meteorites and exhibited an ee of approximately 15 % (Cronin and Pizzarello 1997; Pizzarello et al 2003; Pizzarello 2006), or 18.5 % (Glavin and Dworkin 2009) affected D,L-Asn and other physiological amino acids during recrystallization.
Materials and Methods
Materials
S- and R-Iva were purchased from Nagase & Co., Ltd. (Tokyo, Japan). D,L-Phe, D,L-glutamine (Gln), D,L-Asp, D,L-His, and N-(5-fluoro-2,4-dinitrophenyl)-L- leucinamide (FDLA) were purchased from Tokyo Chem. Ind. Co., Ltd. (Tokyo, Japan). D,L-Trp and D,L-Tyr were purchased from Nacalai Tesque, Inc. (Kyoto, Japan). Other chemicals were purchased from Wako Pure Chem. Ind., Ltd. (Osaka, Japan).
Recrystallization of D,L-Asn with S-Iva
D,L-Asn monohydrate (13.3 mmol: 2 g) and S-Iva monohydrate (6.7 mmol: 900 mg) were dissolved in 10 ml of water in a boiling water bath. After filtration with a glass filter, the resulting solution was divided in 5 vessels. After recrystallization, crystals were collected from each vessel.
Determination of the Quantity of Asn and S-Iva and the ee of Asn by High-Performance Liquid Chromatography (HPLC)
Crystals from each vessel were collected and dissolved in water to a final concentration of approximately 2 mM, assuming that all crystals were consisted of D,L-Asn. An aliquot of 100 μl was taken out and mixed with 900 μl of 0.1 M sodium bicarbonate. From the solution 200 μl aliquot was taken out in a screw-capped vial and mixed with 200 μl acetone solution of 5 mM FDLA based on the method of Fujii et al (1997). The resulting solution was kept at 40 °C for 1 h for determining Asn and for 24 h for determining S-Iva, because Iva reacts with FDLA much more slowly than Asn. After the reaction completed, 100 μl of 0.2 M HCl solution was added at 0 °C, and the solution was directly applied to a μBondasphere 5C18 column (3.9 × 150 mm, Waters Corp., Milford, MA, USA). A Shimadzu LC-10 AD pump was used. For determining D- and L-Asn, a mixture (0.6 ml/min) of 25 % acetonitrile and 75 % 20 mM sodium acetate solution adjusted to pH 4.0 with acetic acid was used as an eluent. For determining S-Iva, the elution was made with a mixture (0.6 ml/min) of 40 % acetonitrile and 60 % 20 mM sodium acetate solution adjusted to pH 4.0 with acetic acid. The absorption at 340 nm was recorded with a Shimadzu SPD-10A spectrophotometer. In the original report (Fujii et al 1997), D- and L-Asn were not resolved, but they were clearly separated by our HPLC conditions, as described above.
For calibration, 2 mM solutions of D,L-Asn and S-Iva were also treated in a similar manner.
Recrystallization of D,L-Glu with S-Iva
D,L-Glu monohydrate (2 mmol: 330 mg) and S-Iva monohydrate (6.7 mmol: 900 mg) were dissolved in 10 ml of water in a boiling water bath. After filtration with a glass filter, the resulting solution was divided in 5 vessels. After recrystallization, crystals were collected from each vessel.
Determination of the Quantity of Glu and S-Iva and the ee of Glu by HPLC
Crystals from each vessel were dissolved in water to a final concentration of approximately 2 mM, assuming that all crystals were consisted of D,L-Glu. The resulting solution was directly applied to a Crownpak CR(+) column (Daicel Chem. Ind. Ltd., Tokyo, Japan), eluted with an aqueous HClO4 solution (0.4 ml/min) at pH 1.5 at room temperature, and the absorption at 200 nm was recorded. D-Glu, L-Glu, and S-Iva were separated with this chiral column and determinations were made simultaneously.
Recrystallization of D,L-Glu with R-Iva
D,L-Glu monohydrate (2 mmol: 330 mg) and R-Iva monohydrate (6.7 mmol: 900 mg) were dissolved in 10 ml of water in a boiling water bath. After filtration with a glass filter, the resulting solution was divided in 5 vessels. After recrystallization, crystals were collected from each vessel.
Determination of the Quantity of R-Iva and Glu and the ee of Glu by HPLC
Since the peak of D-Glu partially coincided with that of R-Iva with a Crownpak CR(+) chiral column, derivatization with FDLA was applied. Crystals from each vessel were dissolved in water to a final concentration of approximately 2 mM, assuming that all crystals were consisted of D,L-Glu.
An aliquot of 100 μl was taken out and mixed with 900 μl of 0.1 M sodium bicarbonate. From the solution, 200 μl was taken out in a screw-capped vial and mixed with a 200 μl acetone solution of 5 mM FDLA. The resulting solution was kept at 40 °C for 1 h for determining Glu and for 24 h for R-Iva. After the reaction, 100 μl of 0.2 M HCl solution was added at 0 °C and the solution was directly applied to a μBondasphere 5C18 column (3.9 × 150 mm, Waters). A mixture (0.55 ml/min) of 27.5 % acetonitrile and 72.5 % 20 mM sodium acetate solution adjusted to pH 4.0 with acetic acid was used as an eluent for determining D- and L-Glu. For determining R-Iva, a mixture (0.6 ml/min) of 40 % acetonitrile and 60 % 20 mM sodium acetate solution adjusted to pH 4.0 with acetic acid was used as an eluent.
For calibration, 2 mM solutions of D,L-Asn and R-Iva were also treated in a similar manner.
Determination of the ee of Other Amino Acids from Recrystallization with S-Iva
L-Iva fixed at 900 mg and D,L-amino acid were dissolved in 10 ml of water and recrystallization was performed, as described above. The quantity of each D,L-amino acid depended on its solubility. D,L-Amino acids at 400 mg (3 mmol), 1.2 g (10.3 mmol), 600 mg (4 mmol), 220 mg (1.3 mmol), 200 mg (1 mmol), 900 mg (6.2 mmol), 18 mg (0.1 mmol), 150 mg (1.1 mmol), and 500 mg (3.9 mmol) were used for D,L-Leu, D,L-Val, D,L-Met, D,L-Phe, D,L-Trp, D,L-Gln, D,L-Tyr, D,L-Asp, and D,L-His, respectively.
Derivatization with FDLA was used for determining the ee values of Leu, Val, Met, and His. A Crownpak CR(+) column was applied for determining the ee values of Phe, Trp, Gln, Tyr, and Asp.
Results
Firstly we defined ee (%) = 100 × (L − D)/(L + D). Thus, a positive ee value indicates L-rich crystals, and a negative ee value indicates D-rich crystals. Usually, negative ee values are not used, but it is necessary for statistical analysis in this study. The ee value was shown as a mean ± SD (standard deviation). It is generally assumed that the detection limit of the ee by the chromatographic method including HPLC and gas chromatography is approximately 1 %.
To detect the effect of Iva, a very high concentration of Iva monohydrate (900 mg/10 ml = 0.67 M) was applied.
Recrystallization of D,L-Glu with S-Iva or R-Iva
D,L-Glu monohydrate (330 mg) alone was dissolved in 10 ml of hot water. After filtration, the resulting solution was divided in 5 vessels. After recrystallization, crystals were collected from each vessel, and their ee values were determined as −0.03, 0.2, 0.7, 0.7 and 0.8 % (ee was 0.474 % ± 0.33 %).
D,L-Glu monohydrate (330 mg) and S-Iva monohydrate (900 mg) were dissolved in 10 ml of hot water. After filtration, the resulting solution was divided in 5 vessels. After recrystallization, crystals were collected from each vessel, and their ee values were determined. Results are shown in Table 1. The ee values of Glu were all negative, and the mean and SD was −2.69 % ± 0.77 % (n = 10). It was possible that this value was an artifact. Therefore, recrystallizations of D,L-Glu with R-Iva were performed as well, and the results are shown in Table 2. The ee values of Glu were all positive, and their mean and SD was 6.24 % ± 2.1 % (n = 10). The differences among these three ee values were statistically significant by the Tukey-Kramer method (p < 0.01).
Recrystallization of D,L-Asn with S-Iva
Recrystallizations of D,L-Asn provided random ee values of 4.8, 11.2, 11.5, 11.7, 15.5, 26.7, 26.8, 27.1, 64.7, 88.9, and −59.7 % (Kojo and Tanaka 2001). Recrystallizations of D,L-Asn in the presence of S-Iva also provided random ee values of Asn at −44.5, −7.8, −2.8, 1.8, 3.7, 4.4, 6.1, 8.5, 19.4 and 84 %, showing no effect of S-Iva on the ee of Asn.
Recrystallization of Other D,L-Amino Acids with S-Iva
The ee values of crystals obtained from other D,L-amino acids with S-Iva were 0.22 % ± 0.18 % (n = 5) for D,L-Leu, −0.46 % ± 0.27 % (n = 5) for D,L-Val, 0.26 % ± 0.36 % (n = 5) for D,L-Phe, 0 % ± 0.34 % (n = 5) for D,L-Trp, 1.54 % ± 3.9 % (n = 5) for D,L-Gln, and −0.16 % ± 0.53 % (n = 5) for D,L-Tyr, demonstrating that S-Iva did not induce any significant differences in ee values for these amino acids.
The ee value of crystals obtained from the recrystallizations of D,L-Met with S-Iva was 1.14 % ± 0.90 % (n = 10), and that from similar recrystallizations using R-Iva was 0.5 % ± 0.74 % (n = 5). These values were not statistically significant, and it was concluded that S-Iva did not cause any effect on the ee of D,L-Met.
The ee of crystals obtained from the recrystallizations of D,L-Asp alone was −0.56 % ± 0.30 % (n = 5). The ee of crystals obtained from the recrystallizations of D,L-Asp with S-Iva was −1.96 % ± 0.88 % (n = 5), while that obtained using R-Iva was −0.34 % ± 0.30 % (n = 5). Although the difference between these two induced ee values was statistically significant, the ee of −0.34 % ± 0.30 % (obtained from the recrystallizations of D,L-Asp with R-Iva) was not significantly different from the original ee of −0.56 ± 0.30 % (obtained from the recrystallizations of D,L-Asp alone), indicating that R-Iva had no clear effect on the ee of D,L-Asp. Thus, it was concluded that S-Iva did not influence the ee of D,L-Asp.
The ee value of crystals obtained from the recrystallizations of D,L-His alone was 0.12 % ± 0.24 % (n = 5). The ee of crystals obtained from the recrystallizations of D,L-His with S-Iva was 1.48 % ± 0.28 % (n = 10), while that obtained using R-Iva was 0.28 % ± 0.22 % (n = 5). Although the difference between these two induced results are statistically significant, the ee of 0.28 % ± 0.22 % (obtained from the recrystallizations of D,L-His with R-Iva) was not significantly different from the original ee of 0.12 % ± 0.24 % (obtained from the recrystallizations of D,L-His alone), demonstrating that R-Iva did not affect the ee of D,L-His. Considering that the ee in the case using R-Iva was smaller than 1 %, which was the detection limit of ee utilizing the chromatographic method, it was concluded that S-Iva did not affect the ee of D,L-His.
Recrystallization of other D,L-amino acids with S-Iva was not examined because of their high solubility in water (alanine, Arg, proline, Ser, and lysine), oxidizable nature (cysteine), or having two chiral carbons (i.e., threonine and isoleucine).
Discussion
D,L-Asn is the only amino acid known to induce ee when co-existing with other D,L-amino acids with the same configuration as Asn during recrystallization (Kojo et al 2004). This induction of ee from racemic amino acids is very specific to Asn and this chiral generation must be strictly distinguished from chiral amplification. D,L-Asn is racemic, but when L- or D-Asn crystallizes, its surface should have a strong asymmetric field. These results indicate that recrystallization is a mechanism that leads to a set of L- or D-amino acids. However, it was incidental whether the enrichment occurred with L- or D-Asn. Although it is possible that the homochirality of amino acids was generated incidentally, it is desirable to find a condition where L-amino acids necessarily generate. We examined whether it was possible to make L-enrichment selective by interaction with other asymmetric molecules.
We firstly examined isovaline, which was contained in meteorites with an ee of approximately 15 % (Pizzarello et al 2003), or 18.5 % (Glavin and Dworkin 2009). In prebiotic earth, amino acids generated by chemical evolution must have been racemic, and amino acids in meteorites may have been the only source of nonracemic molecules. Regrettably, S-Iva did not affect the ee of D,L-Asn, the key amino acid, during recrystallization. However, S-Iva did cause a significant negative ee, i.e., −2.69 ± 0.77 %, in D,L-Glu during co-crystallization. This is the first finding that S-Iva induced a significant change in the ee of a physiological amino acid.
The results of Tables 1 and 2 demonstrate that Iva was more soluble in water than Glu, as evidenced by its lower content in crystals than D,L-Glu. In addition, S-Iva had a greater affinity to L-Glu than to D-Glu, as more L-Glu interacted with S-Iva and remained in the solution, resulting in D-rich crystals. As the mirror image, R-Iva exhibited more affinity to D-Glu than to L-Glu, as shown in Table 2.
Since S-Iva did not induce changes in ee in other D,L-amino acids, the ability of S-Iva to induce ee changes in other amino acids appeared less prominent than that of Asn, despite the use of pure S-Iva at concentrations as high as 0.67 M.
On the other hand meteorites contained significant amount of Asp with a high ee (Glavin et al 2012; Burton et al 2013) and it was possible that some part of the Asp was derived from Asn by acid hydrolysis during analysis. Therefore the involvement of Asn in the origin of homochirality of amino acids is still a probable mechanism.
Abbreviations
- Arg:
-
Arginine
- Asn:
-
Asparagine
- Asp:
-
Aspartic acid
- ee:
-
Enantiomeric excess
- FDLA:
-
N-(5-fluoro-2,4-dinitrophenyl)-L-leucinamide
- Gln:
-
Glutamine
- Glu:
-
Glutamic acid
- His:
-
Histidine
- HPLC:
-
High-performance liquid chromatography
- Iva:
-
Isovaline
- Leu:
-
Leucine
- Met:
-
Methionine
- Phe:
-
Phenylalanine
- SD:
-
Standard deviation
- Ser:
-
Serine
- Trp:
-
Tryptophan
- Tyr:
-
Tyrosine
- Val:
-
Valine
References
Avalos M, Babiano R, Cintas P, Jimenez, Palacios JC (2004) Symmetry breaking by spontaneous crystallization—is it the most plausible source of terrestrial handedness we have long been looking for?—A reappraisal. Orig Life Evol Biosph 34:391–405
Breslow R, Levine MS (2006) Amplification of enantiometric concentrations under credible prebiotic conditions. Proc Natl Acad Sci U S A 103:12979–12980
Burton AS, Elsila JE, Hein JE, Glavin DP, Dworkin JP (2013) Extraterrestrial amino acids identified in metal-rich CH and CB carbonaceous chondrites from Antarctica. Meteorit Planet Sci 48:390–402
Cronin JR, Pizzarello S (1997) Enantiomeric excess in meteoritic amino acids. Science 275:951–955
Fujii K, Ikai Y, Oka H, Suzuki M, Harada K (1997) A nonempirical method using LC/MS for determination of the absolute configuration of constituent amino acids in a peptide: combination of Marfey’s method with mass spectrometry and its practical application. Anal Chem 69:5146–5151
Glavin DP, Dworkin JP (2009) Enrichment of the amino acid L-isovaline by aqueous alteration on CI and CM meteorite parent bodies. Proc Natl Acad Sci U S A 106:5487–5492
Glavin DP, Elsila JE, Burton AS, Callahan MP, Dworkin JP, Hilts RW, Herd CDK (2012) Unusual nonterrestrial L-proteinogenic amino acid excesses in the Tagish Lake meteorite. Meteorit Planet Sci 47:1347–1364
Kojo S (2010) Origin of homochirality of amino acids in the biosphere. Symmetry 2:1022–1032
Kojo S, Tanaka K (2001) Enantioselective crystallization of D,L-amino acids induced by spontaneous asymmetric resolution of D,L-asparagine. Chem Comm 1980–1981. doi:10.1039/B105663H
Kojo S, Uchino H, Yoshimura M, Tanaka K (2004) Racemic D,L-asparagine causes enantiomeric excess of other coexisting racemic D,L-amino acids during recrystallization: a hypothesis accounting for the origin of L-amino acids in the biosphere. Chem Comm 2146–2147
Pizzarello S (2006) The chemistry of life’s origin: a carbonaceous meteorite perspective. Acc Chem Res 39:231–237
Pizzarello S, Zolensky M, Turk KA (2003) Nonracemic isovaline in the Murchinson meteorite: chiral distribution and mineral association. Geochim Cosmochim Acta 67:1589–1595
Soai K, Shibata T, Morioka H, Choji K (1995) Asymmetric autocatalysis and amplification of enantiomeric excess of a chiral molecule. Nature 378:767–768
Acknowledgments
This study was supported by The Open University of Japan and the Ministry of Education, Culture, Sports, Science and Technology, Japan.
Conflict of Interest
We declare no conflicts of interest in this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kojo, S. S-Isovaline Contained in Meteorites, Induces Enantiomeric Excess in D,L-glutamic Acid During Recrystallization. Orig Life Evol Biosph 45, 85–91 (2015). https://doi.org/10.1007/s11084-015-9407-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11084-015-9407-8