Abstract
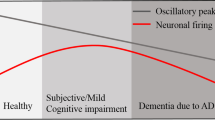
The typical hallmark of electroencephalogram (EEG) in Alzheimer’s disease (AD) is a slowing of rhythms and perturbations in synchrony. However, the mechanism of AD electrophysiological abnormalities is still ambiguous. Synapse deficiency has been considered as an evident neuropathological change in AD that is closely associated with cognitive decline. The main purpose of this work is to explore how synapse deficiency in AD affects these electrophysiological features using neural computational techniques. First, based on the Diffusion Tensor Imaging data, a connectivity matrix of a structural brain network is constructed by means of a pipeline toolbox called PANDA. Using this data-driven connectivity matrix, a cortical network model with 90 cortical areas is then be built in which each cortical area is modeled by a neuron mass model. Subsequently, by reducing the synaptic strength parameter to mimic synapse deficiency in AD, our results show that the synapse deficiency does not only cause a leftward shift of the dominant frequency, but also induces a decrease in the alpha rhythm and an increase in the theta rhythm. Further, the influence of synapse deficiency on phase synchrony is investigated by the phase lag index (PLI). When the synaptic strength parameter is reduced, the alpha-band PLI decreases and theta-band PLI increases. Moreover, a statistical analysis of the differences between the simulated AD and healthy control (HC) in terms of synchronization and rhythms is performed. The results demonstrate that there are significant differences between simulated AD and HC groups. All the above simulation results are consistent with the EEG changes of AD in the physiological experiments. Finally, a strong statistical correlation between PLI and relative power is revealed using Pearson’s correlation analysis. This study reveals a close relationship between synapse deficiency and electrophysiological abnormalities in AD, which may provide new insight for the early diagnosis of AD.











Similar content being viewed by others
References
Koffie, R.M., Hyman, B.T., Spires-Jones, T.L.: Alzheimer’s disease: synapses gone cold. Mol. Neurodegener. 6(1), 63 (2011). https://doi.org/10.1186/1750-1326-6-63
Lista, S., Hampel, H.: Synaptic degeneration and neurogranin in the pathophysiology of Alzheimer’s disease. Expert Rev. Neurother. 17(1), 47–57 (2017). https://doi.org/10.1080/14737175.2016.1204234
Davies, C.A., Mann, D.M.A., Sumpter, P.Q., Yates, P.O.: A quantitative morphometric analysis of the neuronal and synaptic content of the frontal and temporal cortex in patients with Alzheimer’s disease. J. Neurol. Sci. 78(2), 151–164 (1987). https://doi.org/10.1016/0022-510X(87)90057-8
DeKosky, S.T., Scheff, S.W.: Synapse loss in frontal cortex biopsies in Alzheimer’s disease: correlation with cognitive severity. Ann. Neurol. 27(5), 457–464 (1990). https://doi.org/10.1002/ana.410270502
Terry, R.D., Masliah, E., Salmon, D.P., Butters, N., DeTeresa, R., Hill, R., Katzman, R.: Physical basis of cognitive alterations in Alzheimer’s disease: synapse loss is the major correlate of cognitive impairment. Ann. Neurol. 30(4), 572–580 (1991). https://doi.org/10.1002/ana.410300410
Scheff, S.W., Price, D.A., Schmitt, F.A., Mufson, E.J.: Hippocampal synaptic loss in early Alzheimer’s disease and mild cognitive impairment. Neurobiol. Aging 27(10), 1372–1384 (2006). https://doi.org/10.1016/j.neurobiolaging.2005.09.012
Clare, R., King, V.G., Wirenfeldt, M., Vinters, H.V.: Synapse loss in dementias. J. Neurosci. Res. 88(10), 2083–2090 (2010). https://doi.org/10.1002/jnr.22392
Sriram, S., Natiq, H., Rajagopal, K., Parastesh, F., Jafari, S.: Uncovering the correlation between spindle and ripple dynamics and synaptic connections in a hippocampal-thalamic-cortical model. Int. J. Bifurc. Chaos. 33(9), 23501091–235010930 (2023). https://doi.org/10.1142/S0218127423501092
Foroutannia, A., Nazarimehr, F., Ghasemi, M., Jafari, S.: Chaos in memory function of sleep: a nonlinear dynamical analysis in thalamocortical study. J. Theor. Biol. 528, 110837 (2021). https://doi.org/10.1016/j.jtbi.2021.110837
Yan, L.Y., Zhang, H.Z., Sun, Z.K.: Mechanism analysis for excitatory interneurons dominating poly-spike wave and optimization of electrical stimulation. Chaos (2022). https://doi.org/10.1063/5.0076439
Dauwels, J., Vialatte, F., Cichocki, A.: Diagnosis of Alzheimer’s disease from eeg signals: where are we standing? Curr. Alzheimer Res. 7(6), 487–505 (2010). https://doi.org/10.2174/156720510792231720
Jelic, V., Shigeta, M., Julin, P., Almkvist, O., Winblad, B., Wahlund, L.-O.: Quantitative electroencephalography power and coherence in Alzheimer’s disease and mild cognitive impairment. Dement 7(6), 314–323 (1996). https://doi.org/10.1159/000106897
Moretti, D.V., Babiloni, C., Binetti, G., Cassetta, E., Dal Forno, G., Ferreric, F., Rossini, P.M.: Individual analysis of EEG frequency and band power in mild Alzheimer’s disease. Clin. Neurophysiol. 115(2), 299–308 (2004). https://doi.org/10.1016/S1388-2457(03)00345-6
Czigler, B., Csikós, D., Hidasi, Z., Anna Gaál, Z., Csibri, É., Kiss, É., Molnár, M.: Quantitative EEG in early Alzheimer’s disease patients—power spectrum and complexity features. Int. J. Psychophysiol. 68(1), 75–80 (2008). https://doi.org/10.1016/j.ijpsycho.2007.11.002
Gianotti, L.R.R., Künig, G., Lehmann, D., Faber, P.L., Pascual-Marqui, R.D., Kochi, K., Schreiter-Gasser, U.: Correlation between disease severity and brain electric LORETA tomography in Alzheimer’s disease. Clin. Neurophysiol. 118(1), 186–196 (2007). https://doi.org/10.1016/j.clinph.2006.09.007
Tass, P., Rosenblum, M.G., Weule, J., Kurths, J., Pikovsky, A., Volkmann, J., Schnitzler, A., Freund, H.-J.: Detection of n:m phase locking from noisy data: application to magnetoencephalography. Phys. Rev. Lett. 81(15), 3291–3294 (1998). https://doi.org/10.1103/PhysRevLett.81.3291
Stam, C.J., Nolte, G., Daffertshofer, A.: Phase lag index: assessment of functional connectivity from multi channel EEG and MEG with diminished bias from common sources. Hum. Brain Mapp. 28(11), 1178–1193 (2007). https://doi.org/10.1002/hbm.20346
Hardmeier, M., Hatz, F., Bousleiman, H., Schindler, C., Stam, C.J., Fuhr, P.: Reproducibility of functional connectivity and graph measures based on the phase lag index (PLI) and weighted phase lag index (wPLI) derived from high resolution EEG. PLoS ONE 9(10), e108648 (2014). https://doi.org/10.1371/journal.pone.0108648
Zawiślak-Fornagiel, K., Ledwoń, D., Bugdol, M., Romaniszyn-Kania, P., Małecki, A., Gorzkowska, A., Mitas, A.W.: Specific patterns of coherence and phase lag index in particular regions as biomarkers of cognitive impairment in Parkinson’s disease. Parkinsonism. Relat. D. 111, 105436 (2023). https://doi.org/10.1016/j.parkreldis.2023.105436
Polat, H.: Brain functional connectivity based on phase lag index of electroencephalography for automated diagnosis of schizophrenia using residual neural networks. J. Appl. Clin. Med. Phys. (2023). https://doi.org/10.1002/acm2.14039
Kuang, Y., Wu, Z., Xia, R., Li, X., Liu, J., Dai, Y., et al.: Phase lag index of resting-state EEG for identification of mild cognitive impairment patients with type 2 diabetes. Brain Sci. 12(10), 1399 (2022). https://doi.org/10.3390/brainsci12101399
Engels, M.M.A., Stam, C.J., van der Flier, W.M., Scheltens, P., de Waal, H., van Straaten, E.C.W.: Declining functional connectivity and changing hub locations in Alzheimer’s disease: an EEG study. BMC Neurol. 15(1), 145 (2015). https://doi.org/10.1186/s12883-015-0400-7
Kasakawa, S., Yamanishi, T., Takahashi, T., Ueno, K., Kikuchi, M., & Nishimura, H.: Approaches of phase lag index to EEG signals in Alzheimer’s disease from complex network analysis. In: Innovation in Medicine and Healthcare 2015, pp. 459–468. Springer International Publishing (2016). https://doi.org/10.1007/978-3-319-23024-5_42
Jansen, B.H., Rit, V.G.: Electroencephalogram and visual evoked potential generation in a mathematical model of coupled cortical columns. Biol. Cybern. 73(4), 357–366 (1995). https://doi.org/10.1007/BF00199471
Wendling, F., Bartolomei, F., Bellanger, J.J., Chauvel, P.: Epileptic fast activity can be explained by a model of impaired GABAergic dendritic inhibition. Eur. J. Neurosci. 15(9), 1499–1508 (2002). https://doi.org/10.1046/j.1460-9568.2002.01985.x
David, O., Harrison, L., Friston, K.J.: Modelling event-related responses in the brain. Neuroimage 25(3), 756–770 (2005). https://doi.org/10.1016/j.neuroimage.2004.12.030
Zavaglia, M., Astolfi, L., Babiloni, F., Ursino, M.: The effect of connectivity on EEG rhythms, power spectral density and coherence among coupled neural populations: analysis with a neural mass model. IEEE Trans. Biomed. Eng. 55(1), 69–77 (2008). https://doi.org/10.1109/TBME.2007.897814
Ursino, M., Cona, F., Zavaglia, M.: The generation of rhythms within a cortical region: analysis of a neural mass model. Neuroimage 52(3), 1080–1094 (2010). https://doi.org/10.1016/j.neuroimage.2009.12.084
Liu, S., Wang, Q., Fan, D.: disinhibition-induced delayed onset of epileptic spike-wave discharges in a five variable model of cortex and thalamus. Front. Comput. Neurosci. (2016). https://doi.org/10.3389/fncom.2016.00028
Fan, D., Liu, S., Wang, Q.: Stimulus-induced epileptic spike-wave discharges in thalamocortical model with disinhibition. Sci. Rep. 6(1), 37703 (2016). https://doi.org/10.1038/srep37703
Hou, S., Fan, D., Wang, Q.: Regulating absence seizures by tri-phase delay stimulation applied to globus pallidus internal. Appl. Math. Mech. 43(9), 1399–1414 (2022). https://doi.org/10.1007/s10483-022-2896-7
Yan, L., Zhang, H., Sun, Z., Liu, S., Liu, Y., Xiao, P.: Optimization of stimulation waveforms for regulating spike-wave discharges in a thalamocortical model. Chaos Solitons Fractals 158, 112025 (2022). https://doi.org/10.1016/j.chaos.2022.112025
Li, X., Yang, X., Sun, Z.: Alpha rhythm slowing in a modified thalamo-cortico-thalamic model related with Alzheimer’s disease. PLoS ONE 15(3), e0229950 (2020). https://doi.org/10.1371/journal.pone.0229950
Yang, H., Yang, X., Yan, S., Sun, Z.: Effect of acetylcholine deficiency on neural oscillation in a brainstem-thalamus-cortex neurocomputational model related with Alzheimer’s disease. Sci. Rep. 12(1), 14961 (2022). https://doi.org/10.1038/s41598-022-19304-3
Yan, S., Yang, X., Yang, H., Sun, Z.: Decreased coherence in the model of the dorsal visual pathway associated with Alzheimer’s disease. Sci. Rep. 13(1), 3495 (2023). https://doi.org/10.1038/s41598-023-30535-w
Cardenas, V.A., Tosun, D., Yaffe, K.: Co-analysis of structural imaging and DTI in Alzheimer's disease. Proc. Intl. Soc. Mag. Reson. Med. 18 (2010)
Cui, Z., Zhong, S., Xu, P., He, Y., Gong, G.: PANDA: a pipeline toolbox for analyzing brain diffusion images. Front. Hum. Neurosci. (2013). https://doi.org/10.3389/fnhum.2013.00042
Mori, S., Crain, B.J., Chacko, V.P., van Zijl, P.C.: Three-dimensional tracking of axonal projections in the brain by magnetic resonance imaging. Ann. Neurol. 45, 265–269 (1999). https://doi.org/10.1002/1531-8249(199902)45:2%3c265::AID-ANA21%3e3.0.CO;2-3
Tzourio-Mazoyer, N., Landeau, B., Papathanassiou, D., Crivello, F., Etard, O., Delcroix, N., Mazoyer, B., Joliot, M.: Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage 15, 273–289 (2002). https://doi.org/10.1006/nimg.2001.0978
Li, Y., Liu, Y., Li, J., Qin, W., Li, K., Yu, C., Jiang, T.: Brain anatomical network and intelligence. PLoS Comput. Biol. 5(5), e1000395 (2009). https://doi.org/10.1371/journal.pcbi.1000395
Shu, N., Liu, Y., Li, J., Li, Y., Yu, C., Jiang, T.: Altered anatomical network in early blindness revealed by diffusion tensor tractography. PLoS ONE 4(9), e7228 (2009). https://doi.org/10.1371/journal.pone.0007228
Jansen, B.H., Zouridakis, G., Brandt, M.E.: A neurophysiologically-based mathematical model of flash visual evoked potentials. Biol. Cybern.Cybern. 68(3), 275–283 (1993). https://doi.org/10.1007/BF00224863
Zavaglia, M., Astolfi, L., Babiloni, F., Ursino, M.: A neural mass model for the simulation of cortical activity estimated from high resolution EEG during cognitive or motor tasks. J. Neurosci. MethodsMethods 157(2), 317–329 (2006). https://doi.org/10.1016/j.jneumeth.2006.04.022
Sotero, R.C., Trujillo-Barreto, N.J., Iturria-Medina, Y., Carbonell, F., Jimenez, J.C.: Realistically coupled neural mass models can generate EEG rhythms. Neural Comput. 19(2), 478–512 (2007). https://doi.org/10.1162/neco.2007.19.2.478
Penttilä, M., Partanen, J.V., Soininen, H., Riekkinen, P.J.: Quantitative analysis of occipital EEG in different stages of Alzheimer’s disease. EEG Clin. Neurophysiol. 60(1), 1–6 (1985). https://doi.org/10.1016/0013-4694(85)90942-3
Prinz, P.N., Vitiell, M.V.: Dominant occipital (alpha) rhythm frequency in early stage Alzheimer’s disease and depression. EEG Clin. Neurophysiol. 73(5), 427–432 (1989). https://doi.org/10.1016/0013-4694(89)90092-8
Babiloni, C., Arakaki, X., Azami, H., Bennys, K., Blinowska, K., Bonanni, L., et al.: Measures of resting state EEG rhythms for clinical trials in Alzheimer’s disease: Recommendations of an expert panel. Alzheimer’s Dement. 17(9), 1528–1553 (2021). https://doi.org/10.1002/alz.12311
Del Percio, C., Lopez, S., Noce, G., Lizio, R., Tucci, F., Soricelli, A., et al.: What a single electroencephalographic (EEG) channel can tell us about Alzheimer’s disease patients with mild cognitive impairment. Clin. EEG Neurosci. 54(1), 21–35 (2023). https://doi.org/10.1016/j.ijpsycho.2022.10.011
Acknowledgements
This work is partially supported by the National Natural Science Foundation of China (Grant Nos. 11972217, 12372062). JK acknowledges support from the Ministry of Science and Higher Education of the Russian Federation within the framework of state support for the creation and development of World-Class Research Centers “Digital biodesign and personalized healthcare” (No. 075-15-2020-926).
Funding
This work is funded by the National Natural Science Foundation of China (Grant Nos. 11972217, 12372062). JK acknowledges support from the Ministry of Science and Higher Education of the Russian Federation within the framework of state support for the creation and development of World-Class Research Centers “Digital biodesign and personalized healthcare” (No. 075-15-2020-926).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Data collection and analysis were performed by SY and XY. The first draft of the manuscript was written by SY and XY. JK proposed constructive advice and polished the language. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest or competing interests.
Ethics approval
Not applicable.
Availability of data and code
The data and code used and analysed during the current study are available from the corresponding author on reasonable request.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yan, S., Yang, X. & Kurths, J. Abnormalities of rhythms and phase lag index in the data-driven cortical network model of Alzheimer's disease. Nonlinear Dyn 111, 21289–21306 (2023). https://doi.org/10.1007/s11071-023-08968-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11071-023-08968-9