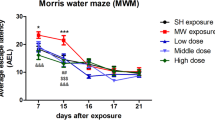
Abstract
Microwave radiation (MWR) has been linked to neurodegeneration by inducing oxidative stress in the hippocampus of brain responsible for learning and memory. Ashwagandha (ASW), a medicinal plant is known to prevent neurodegeneration and promote neuronal health. This study investigated the effects of MWR and ASW on oxidative stress and cholinergic imbalance in the hippocampus of adult male Japanese quail. One control group received no treatment, the second group quails were exposed to MWR at 2 h/day for 30 days, third was administered with ASW root extract orally 100 mg/day/kg body weight and the fourth was exposed to MWR and also treated with ASW. The results showed that MWR increased serum corticosterone levels, disrupted cholinergic balance and induced neuro-inflammation. This neuro-inflammation further led to oxidative stress, as evidenced by decreased activity of antioxidant enzymes SOD, CAT and GSH. MWR also caused a significant decline in the nissil substances in the hippocampus region of brain indicating neurodegeneration through oxidative stress mediated hippocampal apoptosis. ASW, on the other hand, was able to effectively enhance the cholinergic balance and subsequently lower inflammation in hippocampus neurons. This suggests that ASW can protect against the neurodegenerative effects of MWR. ASW also reduced excessive ROS production by increasing the activity of ROS-scavenging enzymes. Additionally, ASW prevented neurodegeneration through decreased expression of caspase-3 and caspase-7 in hippocampus, thus promoting neuronal health. In conclusion, this study showed that MWR induces apoptosis and oxidative stress in the brain, while ASW reduces excessive ROS production, prevents neurodegeneration and promotes neuronal health.







Similar content being viewed by others
Data Availability
The datasets acquired and/or analyzed during the current study are not publicly available but are available on the reasonable request to corresponding author.
References
Shahin S, Banerjee S, Singh SP, Chaturvedi CM (2015) 2.45 GHz microwave radiation impairs learning and spatial memory via oxidative/nitrosative stress induced p53-dependent/independent hippocampal apoptosis: molecular basis and underlying mechanism. J Toxicol Sci 148:380–399
Hermann DM, Hossmann K-A (1997) Neurological effects of microwave exposure related to mobile communication. J Neurol Sci 152:1–14
Einat M, Yahalom A (2011) Induced static magnetic field by a cellular phone. Appl Phys Lett 99:093503
Shahin S, Banerjee S, Swarup V, Singh SP, Chaturvedi CMJTS (2018) From the cover: 2.45-GHz microwave radiation impairs hippocampal learning and spatial memory: involvement of local stress mechanism-induced suppression of iGluR/ERK/CREB signaling. J Toxicol Sci 161:349–374
Abhold RH, Ortner MJ, Galvin MJ, McRee DI (1981) Studies on acute in vivo exposure of rats to 2450-MHz microwave radiation: II. effects on thyroid and adrenal axes hormones. Radiat Res 88:448–455
Li M, Wang Y, Zhang Y, Zhou Z, Yu Z (2008) Elevation of plasma corticosterone levels and hippocampal glucocorticoid receptor translocation in rats: a potential mechanism for cognition impairment following chronic low-power-density microwave exposure. J Radiat Res 49(2):163–170
Singh KV, Gautam R, Meena R, Nirala JP, Jha SK, Rajamani P (2020) Effect of mobile phone radiation on oxidative stress, inflammatory response, and contextual fear memory in Wistar rat. Environ Sci Pollut Res 27:19340–19351
Megha K, Deshmukh PS, Ravi AK, Tripathi AK, Abegaonkar MP, Banerjee BD (2015) Effect of low-intensity microwave radiation on monoamine neurotransmitters and their key regulating enzymes in rat brain. J Cell Biochem Biophys 73:93–100
Wilson CB, McLaughlin LD, Nair A, Ebenezer PJ, Dange R, Francis J (2013) Inflammation and oxidative stress are elevated in the brain, blood, and adrenal glands during the progression of post-traumatic stress disorder in a predator exposure animal model. PLoS ONE 8:e76146
Xia Q-P, Cheng Z-Y, He LJII (2019) The modulatory role of dopamine receptors in brain neuroinflammation. J Int Immunopharmacol 76:105908
Feng Y, Gao J, Cui Y, Li M, Li R, Cui C, Cui J (2017) Neuroprotective effects of resatorvid against traumatic brain injury in rat: involvement of neuronal autophagy and TLR4 signaling pathway. Cell Mol Neurobiol 37:155–168
Sanchez-Muñoz F, Dominguez-Lopez A, Yamamoto-Furusho JK (2008) Role of cytokines in inflammatory bowel disease. World J Gastroenterol: WJG 14:4280
Avci B, Akar A, Bilgici B, Tunçel ÖK (2012) Oxidative stress induced by 1.8 GHz radio frequency electromagnetic radiation and effects of garlic extract in rats. Int J Radiat Biol 88:799–805
Dasdag S, Bilgin H, Akdag M, Celik H, Aksen FJB (2008) Effect of long term mobile phone exposure on oxidative-antioxidative processes and nitric oxide in rats. J Biotechnol Biotechnol Equip 22:992–997
Megha K, Deshmukh PS, Banerjee BD, Tripathi AK, Abegaonkar MP (2012) Microwave radiation induced oxidative stress, cognitive impairment and inflammation in brain of Fischer rats.
Chauhan P, Verma H, Sisodia R, Kesari KK, medicine, (2017) Microwave radiation (2.45 GHz)-induced oxidative stress: Whole-body exposure effect on histopathology of Wistar rats. J Electromagn Biol 36:20–30
Jaffar FHF, Osman K, Ismail NH, Chin K-Y, Ibrahim SF (2019) Adverse effects of Wi-Fi radiation on male reproductive system: a systematic review. J Tohoku J Exp Med 248:169–179
Kesari KK, Kumar S, Behari J (2011) 900-MHz microwave radiation promotes oxidation in rat brain. Electromagn Biol Med 30:219–234
Butterfield DA, Castegna A, Lauderback CM, Drake J (2002) Evidence that amyloid beta-peptide-induced lipid peroxidation and its sequelae in Alzheimer’s disease brain contribute to neuronal death. J Neurobiol Aging 23:655–664
Zhi W-J, Wang L-F, Hu X-J (2017) Recent advances in the effects of microwave radiation on brains. Mil Med Res 4:1–14
Varghese R, Majumdar A, Kumar G, Shukla A (2018) Rats exposed to 2.45 GHz of non-ionizing radiation exhibit behavioral changes with increased brain expression of apoptotic caspase 3. J Pathophysiology 25:19–30
Kashyap VK, Peasah-Darkwah G, Dhasmana A, Jaggi M, Yallapu MM, Chauhan SC (2022) Withania somnifera: Progress towards a Pharmaceutical Agent for Immunomodulation and Cancer Therapeutics. Pharmaceutics 14:611
Dar NJ, Hamid A, Ahmad M (2015) Pharmacologic overview of Withania somnifera, the Indian Ginseng. Cell Mol Life Sci 72:4445–4460
Mirjalili MH, Moyano E, Bonfill M, Cusido RM, Palazón J (2009) Steroidal lactones from Withania somnifera, an ancient plant for novel medicine. Molecules 14:2373–2393
Gupta V, Srivastava R (2022) 2.45 GHz microwave radiation induced oxidative stress: Role of inflammatory cytokines in regulating male fertility through estrogen receptor alpha in Gallus gallus domesticus. Biochem Biophys Res Commun 629:61–70
Gandhi OP, Hunt EL, D’Andrea JA (1977) Deposition of electromagnetic energy in animals and in models of man with and without grounding and reflector effects. J Radio Sci 12:39–47
Baghel K, Srivastava R (2021) Photoperiod dependent expression of estrogen receptor alpha in testes of Japanese quail: Involvement of Withania somnifera in apoptosis amelioration. J Biochem Biophys Res Commun 534:957–965
Sengupta P, Agarwal A, Pogrebetskaya M, Roychoudhury S, Durairajanayagam D, Henkel R (2018) Role of Withania somnifera (Ashwagandha) in the management of male infertility. Reprod Biomed Online 36:311–326
Dey A, Chatterjee SS, Kumar V (2018) Triethylene glycol-like effects of Ashwagandha (Withania somnifera (L.) Dunal) root extract devoid of withanolides in stressed mice. Ayu 39:230
Stefanini M, Martino CD, Zamboni L (1967) Fixation of ejaculated spermatozoa for electron microscopy. Nature 216:173–174
Baghel K, Niranjan MK, Srivastava R (2023) Withania somnifera inhibits photorefractoriness which triggers neuronal apoptosis in both pre-optic and paraventricular hypothalamic area of Coturnix coturnix japonica: involvement of oxidative stress induced p53 dependent Caspase-3 mediated low immunoreactivity of estrogen receptor alpha. Photochem Photobiol Sci 22(9):2205–2218
Baghel K, Srivastava R (2021) Stress and steroid interaction modulates expression of estrogen receptor alpha in the brain, pituitary, and testes of immature Gallus gallus domesticus. Stress 24:931–944
Lowry O, Rosebrough N, Farr AL, Randall R (1951) Protein measurement with the Folin phenol reagent. J J Biol Chem 193:265–275
Beauchamp CF, Irwin J, Analytical biochemistry, (1971) Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. J Anal Biochem 44:276–287
Yates D (1984) Methods of Enzymatic Analysis: 3rd edn, Volume 1-Edited by J. Bergmeyer and M. Grassl Verlag Chemie; Weinheim. Deerfield Beach, Florida. Basel, 1983 574 pages. DM 230.00, DM 170.00 (subscribers). J FEBS Letters 1:201
Niranjan MK, Koiri RK, Srivastava R (2021) Expression of estrogen receptor alpha in response to stress and estrogen antagonist tamoxifen in the shell gland of Gallus gallus domesticus: Involvement of anti-oxidant system and estrogen. J Stress 24:261–272
Jollow DM, Zampaglione JR, Nal Gillette JR (1974) Bromobenzene-induced liver necrosis. Protective role of glutathione and evidence for 3, 4-bromobenzene oxide as the hepatotoxic metabolite. J Pharmacol 11:151–169
Niranjan MK, Srivastava R (2019) Expression of estrogen receptor alpha in developing brain, ovary and shell gland of Gallus gallus domesticus: Impact of stress and estrogen. J Steroids 146:21–33
Placer ZAC, Johnson LL, Connor B (1966) Estimation of product of lipid peroxidation (malonyl dialdehyde) in biochemical systems. J Anal Biochem 16:359–364
Baghel K, Niranjan MK, Srivastava R (2020) Water and food restriction decreases immunoreactivity of oestrogen receptor alpha and antioxidant activity in testes of sexually mature Coturnix coturnix japonica. J J Animal Physiol Animal Nutr 104:1738–1747
Sinha AK (1972) Colorimetric assay of catalase. J Anal Biochem 47:389–394
Khan A, Kango N, Srivastava R (2024) Impact of dietary probiotics on the immune and reproductive physiology of pubertal male Japanese Quail (Coturnix coturnix japonica) administered at the onset of pre-puberty. Probiotics Antimicrob Proteins. https://doi.org/10.1007/s12602-023-10209-9
Mahajan L, Verma PK, Raina R, Pankaj NK, Sood S, Singh M (2018) Alteration in thiols homeostasis, protein and lipid peroxidation in renal tissue following subacute oral exposure of imidacloprid and arsenic in Wistar rats. Toxicol Rep 5:1114–1119
Ellman GL (1961) A new and rapid colormetric determination of acetylcholinesterase activity. Biochem pharmacol 7:88–95
Ilesanmi OB, Adewunmi R, Taiwo AT, Komolafe KC, Odewale TT, Akinmoladun AC, Olaleye TM, Akindahunsi AA (2019) Alteration of NADH succinate dehydrogenase activity and redox status by different solvent fractions of antiaris africana in the brain of rats exposed to rotenone. Biomed J 1:7
Baghel K, Azam Z, Srivastava R, Gupta N, Kango N (2023) Withaferin-A attenuates diabetes mellitus induced male reproductive dysfunction mediated by ERα in brain and testes of Swiss albino mice. Sci Rep 13:17625
Singh KB, Maurya BK, Trigun SK (2015) Activation of oxidative stress and inflammatory factors could account for histopathological progression of aflatoxin-B1 induced hepatocarcinogenesis in rat. Mol Cell Biochem 401:185–196
Baghel K, Srivastava R (2020) Effect of estrogen and stress on estrogen receptor 1 in the HPG axis of immature male Gallus gallus domesticus: Involvement of anti-oxidant system. J Theriogenol 155:98–113
Hardell L, Sage C (2008) Biological effects from electromagnetic field exposure and public exposure standards. Biomed Pharmacother 62:104–109
Srivastava KBZAR (2024) Dietary restriction-induced alterations on estrogen receptor alpha expression in regulating fertility in male Coturnix coturnix japonica: Relevance of Withania somnifera in modulation of inflammation and oxidative stress in testis. Am J Reprod Immunol 91(2):e13816
Lai H, Carino M, Horita A, Guy A (1993) Effects of a 60 Hz magnetic field on central cholinergic systems of the rat. Bioelectromagnetics 14:5–15
Fowler CJ, Benedetti MS (1983) The metabolism of dopamine by both forms of monoamine oxidase in the rat brain and its inhibition by cimoxatone. J Neurochem 40:1534–1541
Ciarmiello A (2011) Imaging of neuroinflammation. Eur J Nucl Med Mol Imaging 38:2198–2201
Li Z, Peng RY, Wang SM, Wang LF, Gao YB, Ji D, Xiang L, Su ZT (2012) Relationship between cognition function and hippocampus structure after long-term microwave exposure. Biomed Environ Sci 25:182–188
Gupta M, Kaur G (2018) Withania somnifera as a potential anxiolytic and anti-inflammatory candidate against systemic lipopolysaccharide-induced neuroinflammation. NeuroMol Med 20:343–362
Kaur T, Singh H, Mishra R, Manchanda S, Gupta M, Saini V, Sharma A, Kaur G (2017) Withania somnifera as a potential anxiolytic and immunomodulatory agent in acute sleep deprived female Wistar rats. Mol Cell Biochem 427:91–101
Hosny EN, El-Gizawy MM, Sawie HG, Abdel-Wahhab KG, Khadrawy YA (2021) Neuroprotective Effect of Ashwagandha Extract against the Neurochemical Changes Induced in Rat Model of Hypothyroidism. J J Diet Suppl 18:72–91
Nazıroğlu M, Yüksel M, Köse SA, Özkaya MO (2013) Recent reports of Wi-Fi and mobile phone-induced radiation on oxidative stress and reproductive signaling pathways in females and males. J J Membr Biol 246:869–875
Berköz M, Arslan B, Yıldırım M, Aras N, Yalın S, Çömelekoğlu Ü (2018) 1800 MHz radio-frequency electromagnetic radiation induces oxidative stress in rat liver, kidney and brain tissues. East J Med 23:71
M Ali H (2021) Ashwagandha (Withania somnifera) and Their Effects on the Reproductive Hormones of Male Rats. 37:1–22
Shukla KK, Mahdi AA, Mishra V, Rajender S, Sankhwar SN, Patel D, Das M (2011) Withania somnifera improves semen quality by combating oxidative stress and cell death and improving essential metal concentrations. Reprod Biomed Online 22:421–427
Tan S, Wang H, Xu X, Zhao L, Zhang J, Dong J, Yao B, Wang H, Zhou H, Gao YJSr, (2017) Study on dose-dependent, frequency-dependent, and accumulative effects of 1.5 GHz and 2.856 GHz microwave on cognitive functions in Wistar rats. J Sci Rep 7:1–13
Xiong L, Sun CF, Zhang J, Gao YB, Wang LF, Zuo HY, Wang SM, Zhou HM, Xu XP, Ji D (2015) Microwave exposure impairs synaptic plasticity in the rat hippocampus and PC12 cells through over-activation of the NMDA receptor signaling pathway. Biomed Environ Sci 28:13–24
Funding
The authors would like to express their gratitude to Dr. Harisingh Gour Central University, Sagar (M.P.), India, and UGC, India, for supporting Vaibhav Gupta financially through a non-NET research fellowship.
Author information
Authors and Affiliations
Contributions
VG: Writing- original draft, methodology, figures and formal analysis. RS: Conceptualization, visualization, review, editing and supervision.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Conflict of interest
All the authors indicate no financial, legal or professional conflict of interest.
Ethical Approval
For the goal of maintaining control and oversight of experimental animals, all experimental procedures for this study were carried out in accordance with the regulations and standards of the animal ethics committee (CPCSEA). Additionally, Institutional Animal Ethics Committee (IAEC) of Dr. Harisingh Gour Vishwavidyalaya Sagar (M.P.) also authorized the complete experimental design, as evidenced by certificate No. 379/CPCSEA/IAEC-2021/018.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gupta, V., Srivastava, R. Ashwagandha Diminishes Hippocampal Apoptosis Induced by Microwave Radiation by Acetylcholinesterase Dependent Neuro-Inflammatory Pathway in Male Coturnix coturnix Japonica. Neurochem Res (2024). https://doi.org/10.1007/s11064-024-04127-7
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11064-024-04127-7