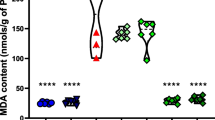
Abstract
Iron overload-induced oxidative stress is implicated in various neurodegenerative disorders. Given the numerous adverse effects associated with current iron chelators, natural antioxidants are being explored as alternative therapeutic options. Dithiolethiones found in cruciferous vegetables have emerged as promising candidates against a wide range of toxicants owing to their lipophilic and cytoprotective properties. Here, we test the dithiolethiones 3H-1,2-dithiole-3-thione (D3T) and 5-amino-3-thioxo-3H-(1,2) dithiole-4-carboxylic acid ethyl ester (ACDT) against ferric ammonium citrate (FAC)-induced toxicity in U-87 MG astrocytoma cells. Exposure to 15 mM FAC for 24 h resulted in 54% cell death. A 24-h pretreatment with 50 μM D3T and ACDT prevented this cytotoxicity. Both dithiolethiones exhibited antioxidant effects by activating the nuclear factor erythroid 2-related factor-2 (Nrf2) transcription factor and upregulating levels of intracellular glutathione (GSH). This resulted in the successful inhibition of FAC-induced reactive oxygen species, lipid peroxidation, and cell death. Additionally, D3T and ACDT upregulated expression of the Nrf2-mediated iron storage protein ferritin which consequently reduced the total labile iron pool. A 24-h pretreatment with D3T and ACDT also prevented cell death induced by the ferroptosis inducer erastin by upregulating the transmembrane cystine/glutamate antiporter (xCT) expression. The resulting increase in intracellular GSH and alleviation of lipid peroxidation was comparable to that caused by ferrostatin-1, a specific ferroptosis inhibitor. Collectively, our findings demonstrate that dithiolethiones may show promise as potential therapeutic options for the treatment of iron overload disorders.





Similar content being viewed by others
Data Availability
Enquiries about data availability should be directed to the corresponding author.
References
Youssef LA, Spitalnik SL (2017) Iron: a double-edged sword. Transfusion 57(10):2293–2297. https://doi.org/10.1111/trf.14296
Jaishankar M, Tseten T, Anbalagan N, Mathew BB, Beeregowda KN (2014) Toxicity, mechanism and health effects of some heavy metals. Interdiscip Toxicol 7(2):60–72. https://doi.org/10.2478/intox-2014-0009
Li K, Reichmann H (2016) Role of iron in neurodegenerative diseases. J Neural Transm (Vienna) 123(4):389–399. https://doi.org/10.1007/s00702-016-1508-7
Sian-Hulsmann J, Mandel S, Youdim MB, Riederer P (2011) The relevance of iron in the pathogenesis of Parkinson’s disease. J Neurochem 118(6):939–957. https://doi.org/10.1111/j.1471-4159.2010.07132.x
Gordon N (2000) Friedreich’s ataxia and iron metabolism. Brain Dev 22(8):465–468. https://doi.org/10.1016/s0387-7604(00)00175-3
Allaman I, Belanger M, Magistretti PJ (2011) Astrocyte-neuron metabolic relationships: for better and for worse. Trends Neurosci 34(2):76–87. https://doi.org/10.1016/j.tins.2010.12.001
Phatnani H, Maniatis T (2015) Astrocytes in neurodegenerative disease. Cold Spring Harb Perspect Biol. https://doi.org/10.1101/cshperspect.a020628
Reinert A, Morawski M, Seeger J, Arendt T, Reinert T (2019) Iron concentrations in neurons and glial cells with estimates on ferritin concentrations. BMC Neurosci 20(1):25. https://doi.org/10.1186/s12868-019-0507-7
Bishop GM, Dang TN, Dringen R, Robinson SR (2011) Accumulation of non-transferrin-bound iron by neurons, astrocytes, and microglia. Neurotox Res 19(3):443–451. https://doi.org/10.1007/s12640-010-9195-x
Gaasch JA, Lockman PR, Geldenhuys WJ, Allen DD, Van der Schyf CJ (2007) Brain iron toxicity: differential responses of astrocytes, neurons, and endothelial cells. Neurochem Res 32(7):1196–1208. https://doi.org/10.1007/s11064-007-9290-4
Ansari MI, Khan MM, Saquib M, Khatoon S, Hussain MK (2018) Dithiolethiones: a privileged pharmacophore for anticancer therapy and chemoprevention. Future Med Chem 10(10):1241–1260. https://doi.org/10.4155/fmc-2017-0281
Munday R, Zhang Y, Paonessa JD, Munday CM, Wilkins AL, Babu J (2010) Synthesis, biological evaluation, and structure-activity relationships of dithiolethiones as inducers of cytoprotective phase 2 enzymes. J Med Chem 53(12):4761–4767. https://doi.org/10.1021/jm100425v
Kwak MK, Egner PA, Dolan PM, Ramos-Gomez M, Groopman JD, Itoh K et al (2001) Role of phase 2 enzyme induction in chemoprotection by dithiolethiones. Mutat Res 480–481:305–315. https://doi.org/10.1016/s0027-5107(01)00190-7
Cui Y, Ma S, Zhang C, Li D, Yang B, Lv P et al (2018) Pharmacological activation of the Nrf2 pathway by 3H–1, 2-dithiole-3-thione is neuroprotective in a mouse model of Alzheimer disease. Behav Brain Res 336:219–226. https://doi.org/10.1016/j.bbr.2017.09.011
Kuo PC, Brown DA, Scofield BA, Yu IC, Chang FL, Wang PY et al (2016) 3H–1,2-dithiole-3-thione as a novel therapeutic agent for the treatment of experimental autoimmune encephalomyelitis. Brain Behav Immun 57:173–186. https://doi.org/10.1016/j.bbi.2016.03.015
Kuo PC, Yu IC, Scofield BA, Brown DA, Curfman ET, Paraiso HC et al (2017) 3H–1,2-Dithiole-3-thione as a novel therapeutic agent for the treatment of ischemic stroke through Nrf2 defense pathway. Brain Behav Immun 62:180–192. https://doi.org/10.1016/j.bbi.2017.01.018
Zhang C, Xie L, Guan F, Cui Y (2018) 3H–1,2-dithiole-3-thione protects PC12 cells against amyloid beta 1–42 (Abeta1-42) induced apoptosis via activation of the ERK1/2 pathway. Life Sci 213:74–81. https://doi.org/10.1016/j.lfs.2018.10.025
Kuo PC, Brown DA, Scofield BA, Paraiso HC, Wang PY, Yu IC et al (2018) Dithiolethione ACDT suppresses neuroinflammation and ameliorates disease severity in experimental autoimmune encephalomyelitis. Brain Behav Immun 70:76–87. https://doi.org/10.1016/j.bbi.2018.03.010
Betharia S, Rondomicronn-Ortiz AN, Brown DA (2019) Disubstituted dithiolethione ACDT exerts neuroprotective effects against 6-hydroxydopamine-induced oxidative stress in SH-SY5Y cells. Neurochem Res 44(8):1878–1892. https://doi.org/10.1007/s11064-019-02823-3
Kulkarni N, Gadde R, Gugnani KS, Vu N, Yoo C, Zaveri R et al (2021) Neuroprotective effects of disubstituted dithiolethione ACDT against manganese-induced toxicity in SH-SY5Y cells. Neurochem Int 147:105052. https://doi.org/10.1016/j.neuint.2021.105052
Eid R, Arab NT, Greenwood MT (2017) Iron mediated toxicity and programmed cell death: a review and a re-examination of existing paradigms. Biochim Biophys Acta Mol Cell Res 1864(2):399–430. https://doi.org/10.1016/j.bbamcr.2016.12.002
Karim A, Bajbouj K, Shafarin J, Qaisar R, Hall AC, Hamad M (2022) Iron overload induces oxidative stress, cell cycle arrest and apoptosis in chondrocytes. Front Cell Dev Biol. 10:821014. https://doi.org/10.3389/fcell.2022.821014
Hoepken HH, Korten T, Robinson SR, Dringen R (2004) Iron accumulation, iron-mediated toxicity and altered levels of ferritin and transferrin receptor in cultured astrocytes during incubation with ferric ammonium citrate. J Neurochem 88(5):1194–1202. https://doi.org/10.1046/j.1471-4159.2003.02236.x
Geng N, Shi BJ, Li SL, Zhong ZY, Li YC, Xua WL et al (2018) Knockdown of ferroportin accelerates erastin-induced ferroptosis in neuroblastoma cells. Eur Rev Med Pharmacol Sci 22(12):3826–3836. https://doi.org/10.26355/eurrev_201806_15267
Wood MJ, Skoien R, Powell LW (2009) The global burden of iron overload. Hepatol Int 3(3):434–444. https://doi.org/10.1007/s12072-009-9144-z
Yuen HW, Becker W (2022) Iron toxicity. StatPearls. Treasure Island
Schneider SA, Bhatia KP (2013) Excess iron harms the brain: the syndromes of neurodegeneration with brain iron accumulation (NBIA). J Neural Transm (Vienna) 120(4):695–703. https://doi.org/10.1007/s00702-012-0922-8
Mobarra N, Shanaki M, Ehteram H, Nasiri H, Sahmani M, Saeidi M et al (2016) A review on iron chelators in treatment of iron overload syndromes. Int J Hematol Oncol Stem Cell Res 10(4):239–247
Poggiali E, Cassinerio E, Zanaboni L, Cappellini MD (2012) An update on iron chelation therapy. Blood Transfus 10(4):411–422. https://doi.org/10.2450/2012.0008-12
Shen JC, Zhang YC, Zhao MF (2017) Protective effects of deferasirox and N-acetyl-L-cysteine on iron overload-injured bone marrow. Braz J Med Biol Res 50(12):e6087. https://doi.org/10.1590/1414-431X20176087
Sarkar R, Hazra B, Mandal N (2012) Hepatoprotective potential of caesalpinia crista against iron-overload-induced liver toxicity in mice. Evid Based Complement Alternat Med. 2012:896341. https://doi.org/10.1155/2012/896341
Jia Z, Zhu H, Li Y, Misra HP (2009) Cruciferous nutraceutical 3H–1,2-dithiole-3-thione protects human primary astrocytes against neurocytotoxicity elicited by MPTP, MPP(+), 6-OHDA, HNE and acrolein. Neurochem Res 34(11):1924–1934. https://doi.org/10.1007/s11064-009-9978-8
Dong J, Yan D, Chen SY (2011) Stabilization of Nrf2 protein by D3T provides protection against ethanol-induced apoptosis in PC12 cells. PLoS One 6(2):e16845. https://doi.org/10.1371/journal.pone.0016845
Li W, Jiang H, Song N, Xie J (2011) Oxidative stress partially contributes to iron-induced alpha-synuclein aggregation in SK-N-SH cells. Neurotox Res 19(3):435–442. https://doi.org/10.1007/s12640-010-9187-x
Rakshit J, Mallick A, Roy S, Sarbajna A, Dutta M, Bandyopadhyay J (2020) Iron-induced apoptotic cell death and autophagy dysfunction in human neuroblastoma cell line SH-SY5Y. Biol Trace Elem Res 193(1):138–151. https://doi.org/10.1007/s12011-019-01679-6
Codazzi F, Pelizzoni I, Zacchetti D, Grohovaz F (2015) Iron entry in neurons and astrocytes: a link with synaptic activity. Front Mol Neurosci 8:18. https://doi.org/10.3389/fnmol.2015.00018
Linnerbauer M, Rothhammer V (2020) Protective functions of reactive astrocytes following central nervous system insult. Front Immunol. 11:573256. https://doi.org/10.3389/fimmu.2020.573256
Gleixner AM, Posimo JM, Pant DB, Henderson MP, Leak RK (2016) Astrocytes surviving severe stress can still protect neighboring neurons from proteotoxic injury. Mol Neurobiol 53(7):4939–4960. https://doi.org/10.1007/s12035-015-9427-4
Gammella E, Recalcati S, Cairo G (2016) Dual role of ROS as signal and stress agents: iron tips the balance in favor of toxic effects. Oxid Med Cell Longev 2016:8629024. https://doi.org/10.1155/2016/8629024
Li KR, Yang SQ, Gong YQ, Yang H, Li XM, Zhao YX et al (2016) 3H–1,2-dithiole-3-thione protects retinal pigment epithelium cells against ultra-violet radiation via activation of Akt-mTORC1-dependent Nrf2-HO-1 signaling. Sci Rep 6:25525. https://doi.org/10.1038/srep25525
Dong J, Sulik KK, Chen SY (2008) Nrf2-mediated transcriptional induction of antioxidant response in mouse embryos exposed to ethanol in vivo: implications for the prevention of fetal alcohol spectrum disorders. Antioxid Redox Signal 10(12):2023–2033. https://doi.org/10.1089/ars.2007.2019
Cao Z, Hallur S, Qiu HZ, Peng X, Li Y (2004) Induction of endogenous glutathione by the chemoprotective agent, 3H–1,2-dithiole-3-thione, in human neuroblastoma SH-SY5Y cells affords protection against peroxynitrite-induced cytotoxicity. Biochem Biophys Res Commun 316(4):1043–1049. https://doi.org/10.1016/j.bbrc.2004.02.156
Cao Z, Hardej D, Trombetta LD, Trush MA, Li Y (2003) Induction of cellular glutathione and glutathione S-transferase by 3H–1,2-dithiole-3-thione in rat aortic smooth muscle A10 cells: protection against acrolein-induced toxicity. Atherosclerosis 166(2):291–301. https://doi.org/10.1016/s0021-9150(02)00331-3
Aoyama K (2021) Glutathione in the Brain. Int J Mol Sci. https://doi.org/10.3390/ijms22095010
Kerins MJ, Ooi A (2018) The roles of NRF2 in modulating cellular iron homeostasis. Antioxid Redox Signal 29(17):1756–1773. https://doi.org/10.1089/ars.2017.7176
Waldvogel-Abramowski S, Waeber G, Gassner C, Buser A, Frey BM, Favrat B et al (2014) Physiology of iron metabolism. Transfus Med Hemother 41(3):213–221. https://doi.org/10.1159/000362888
Drakesmith H, Nemeth E, Ganz T (2015) Ironing out ferroportin. Cell Metab 22(5):777–787. https://doi.org/10.1016/j.cmet.2015.09.006
Lin JF, Wu CC, Liao YJ, Jakfar S, Tang ZB, Chen JK et al (2019) In vitro and in vivo evaluations of mesoporous iron particles for iron bioavailability. Int J Mol Sci. https://doi.org/10.3390/ijms20215291
Primiano T, Kensler TW, Kuppusamy P, Zweier JL, Sutter TR (1996) Induction of hepatic heme oxygenase-1 and ferritin in rats by cancer chemopreventive dithiolethiones. Carcinogenesis 17(11):2291–2296. https://doi.org/10.1093/carcin/17.11.2291
Tangudu NK, Alan B, Vinchi F, Worle K, Lai D, Vettorazzi S et al (2018) Scavenging reactive oxygen species production normalizes ferroportin expression and ameliorates cellular and systemic iron disbalances in hemolytic mouse model. Antioxid Redox Signal 29(5):484–499. https://doi.org/10.1089/ars.2017.7089
Handa P, Thomas S, Morgan-Stevenson V, Maliken BD, Gochanour E, Boukhar S et al (2019) Iron alters macrophage polarization status and leads to steatohepatitis and fibrogenesis. J Leukoc Biol 105(5):1015–1026. https://doi.org/10.1002/JLB.3A0318-108R
Zhang Y, Munday R (2008) Dithiolethiones for cancer chemoprevention: where do we stand? Mol Cancer Ther 7(11):3470–3479. https://doi.org/10.1158/1535-7163.MCT-08-0625
Hirschhorn T, Stockwell BR (2019) The development of the concept of ferroptosis. Free Radic Biol Med 133:130–143. https://doi.org/10.1016/j.freeradbiomed.2018.09.043
Mou Y, Wang J, Wu J, He D, Zhang C, Duan C et al (2019) Ferroptosis, a new form of cell death: opportunities and challenges in cancer. J Hematol Oncol 12(1):34. https://doi.org/10.1186/s13045-019-0720-y
Jiao L, Li X, Luo Y, Wei J, Ding X, Xiong H et al (2022) Iron metabolism mediates microglia susceptibility in ferroptosis. Front Cell Neurosci 16:995084. https://doi.org/10.3389/fncel.2022.995084
Han C, Liu Y, Dai R, Ismail N, Su W, Li B (2020) Ferroptosis and its potential role in human diseases. Front Pharmacol 11:239. https://doi.org/10.3389/fphar.2020.00239
Song X, Long D (2020) Nrf2 and ferroptosis: a new research direction for neurodegenerative diseases. Front Neurosci 14:267. https://doi.org/10.3389/fnins.2020.00267
Zhu H, Santo A, Jia Z, Robert LY (2019) GPx4 in bacterial infection and polymicrobial sepsis: involvement of ferroptosis and pyroptosis. React Oxyg Species (Apex) 7(21):154–160. https://doi.org/10.20455/ros.2019.835
Miotto G, Rossetto M, Di Paolo ML, Orian L, Venerando R, Roveri A et al (2020) Insight into the mechanism of ferroptosis inhibition by ferrostatin-1. Redox Biol. 28:101328. https://doi.org/10.1016/j.redox.2019.101328
Shibata Y, Yasui H, Higashikawa K, Miyamoto N, Kuge Y (2019) Erastin, a ferroptosis-inducing agent, sensitized cancer cells to X-ray irradiation via glutathione starvation in vitro and in vivo. PLoS One 14(12):e0225931. https://doi.org/10.1371/journal.pone.0225931
Zhang Y, Fan BY, Pang YL, Shen WY, Wang X, Zhao CX et al (2020) Neuroprotective effect of deferoxamine on erastininduced ferroptosis in primary cortical neurons. Neural Regen Res 15(8):1539–1545. https://doi.org/10.4103/1673-5374.274344
Yang WS, SriRamaratnam R, Welsch ME, Shimada K, Skouta R, Viswanathan VS et al (2014) Regulation of ferroptotic cancer cell death by GPX4. Cell 156(1–2):317–331. https://doi.org/10.1016/j.cell.2013.12.010
Fan Z, Wirth AK, Chen D, Wruck CJ, Rauh M, Buchfelder M et al (2017) Nrf2-Keap1 pathway promotes cell proliferation and diminishes ferroptosis. Oncogenesis 6(8):e371. https://doi.org/10.1038/oncsis.2017.65
Chu J, Liu CX, Song R, Li QL (2020) Ferrostatin-1 protects HT-22 cells from oxidative toxicity. Neural Regen Res 15(3):528–536. https://doi.org/10.4103/1673-5374.266060
Acknowledgements
The authors would like to acknowledge Dr. Dennis Brown from Manchester University, College of Pharmacy, Natural & Health Sciences, Fort Wayne, IN, and Dr. Timothy J. Maher from Massachusetts College of Pharmacy and Health Sciences, School of Pharmacy, Boston for their scientific input.
Funding
This study was funded by Massachusetts College of Pharmacy and Health Sciences, School of Pharmacy, Boston, MA.
Author information
Authors and Affiliations
Contributions
NK: conceptualization, methodology, validation, formal analysis, investigation, data curation, writing an initial draft, visualization. RG: validation, formal analysis, investigation, data curation, writing an initial draft. SB: conceptualization, methodology, data curation, writing- editing, critical review, and revision of the manuscript, supervision, project administration, funding acquisition.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kulkarni, N., Gadde, R. & Betharia, S. Dithiolethiones D3T and ACDT Protect Against Iron Overload-Induced Cytotoxicity and Serve as Ferroptosis Inhibitors in U-87 MG Cells. Neurochem Res 48, 2542–2551 (2023). https://doi.org/10.1007/s11064-023-03927-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-023-03927-7