Abstract
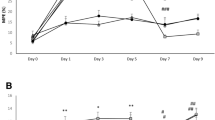
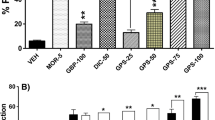
We investigated morphine-induced Straub’s tail reaction (STR) in mice pretreated with or without glycogen synthase kinase-3 (GSK-3) inhibitors (SB216763 and AR-A014418) by using a newly modified, infrared beam sensor-based automated apparatus. Mice treated with a single injection of morphine (30 mg/kg, i.p.) showed a significant STR with a plateau level at a time point of 20 min after morphine challenge. Pretreatment of mice with SB216763 (5 mg/kg, s.c.) or AR-A014418 (3 mg/kg, i.p.) significantly inhibited morphine-induced STR and attenuated the duration of STR in a dose-dependent fashion. In the striatum and the nucleus accumbens, expression of pGSK-3βTyr216 but not GSK3β or pGSK-3βSer9 was largely but not significantly reduced after treatment with SB216763 (5 mg/kg, s.c.) in combination with/without morphine, indicating that the inhibitory effect of GSK-3 inhibitors on morphine-induced STR and hyperlocomotion might not depend on the direct blockade of GSK-3β function. In constipated mice after morphine challenge (30 mg/kg), the effect of GSK-3 inhibitors on gastrointestinal transit was examined to reveal whether the action of GSK-3 inhibitors on morphine effects was central and/or peripheral. Pretreatment with SB216763 (5 mg/kg) did not block constipation in morphine-injected mice. The mechanism of action seems to be central but not peripheral, although the underlying subcellular mechanism of GSK-3 inhibitors is not clear. Our measurement system is a useful tool for investigating the excitatory effects of morphine in experimental animals.




Similar content being viewed by others
Data Availability
The datasets generated during and/or analyzed during the current study that support the findings of this study are available from the corresponding author, Junichi Kitanaka, upon reasonable request.
References
Botz-Zapp CA, Foster SL, Pulley DM, Hempel B, Bi GH, Xi ZX, Newman AH, Weinshenker D, Manvich DF (2021) Effects of the selective dopamine D(3) receptor antagonist PG01037 on morphine-induced hyperactivity and antinociception in mice. Behav Brain Res 415:113506
Varshneya NB, Walentiny DM, Moisa LT, Walker TD, Akinfiresoye LR, Beardsley PM (2021) Fentanyl-related substances elicit antinociception and hyperlocomotion in mice via opioid receptors. Pharmacol Biochem Behav 208:173242
Vashchinkina E, Piippo O, Vekovischeva O, Krupitsky E, Ilyuk R, Neznanov N, Kazankov K, Zaplatkin I, Korpi ER (2018) Addiction-related interactions of pregabalin with morphine in mice and humans: reinforcing and inhibiting effects. Addict Biol 23:945–958
Kitanaka N, Kitanaka J, Hall FS, Kandori T, Murakami A, Muratani K, Nakano T, Uhl GR, Takemura M (2018) Tetrabenazine, a vesicular monoamine transporter-2 inhibitor, attenuates morphine-induced hyperlocomotion in mice through alteration of dopamine and 5-hydroxytryptamine turnover in the cerebral cortex. Pharmacol Biochem Behav 172:9–16
Straub W (1911) Eine empfindliche biologische Reaktion auf Morphin. Deutsche Med Wochenschr 37:1462–1468
Aceto MD, McKean DB, Pearl J (1969) Effects of opiates and opiate antagonists on the Straub tail reaction in mice. Br J Pharmacol 36:225–239
Zarrindast MR, Alaei-Nia K, Shafizadeh M (2001) On the mechanism of tolerance to morphine-induced Straub tail reaction in mice. Pharmacol Biochem Behav 69:419–424
Nath C, Gupta MB, Patnaik GK, Dhawan KN (1994) Morphine-induced straub tail response: mediated by central mu2-opioid receptor. Eur J Pharmacol 263:203–205
Jansen van't Land C, Hendriksen CF (1995) Change in locomotor activity pattern in mice: a model for recognition of distress? Lab Anim 29:286–293
Miyazaki Y, Kobayashi K, Matsushita S, Shimizu N, Murata T (2022) An assessment of the spontaneous locomotor activity of BALB/c mice. J Pharmacol Sci 149:46–52
Kitanaka J, Kitanaka N, Hall FS, Uhl GR, Tanaka K, Nishiyama N, Takemura M (2012) Straub tail reaction in mice treated with σ(1) receptor antagonist in combination with methamphetamine. Brain Res 1482:40–46
Narita M, Suzuki T, Misawa M, Nagase H (1993) Antagonism of the morphine-induced Straub tail reaction by kappa-opioid receptor activation in mice. Psychopharmacology 110:254–256
Johnson SW, North RA (1992) Opioids excite dopamine neurons by hyperpolarization of local interneurons. J Neurosci 12:483–488
Embi N, Rylatt DB, Cohen P (1980) Glycogen synthase kinase-3 from rabbit skeletal muscle. Separation from cyclic-AMP-dependent protein kinase and phosphorylase kinase. Eur J Biochem 107:519–527
Shin K, Kim KH, Yoon MS, Suh DS, Lee JY, Kim A, Eo W (2016) Expression of interactive genes associated with apoptosis and their prognostic value for ovarian serous adenocarcinoma. Adv Clin Exp Med 25:513–521
Beurel E, Grieco SF, Jope RS (2015) Glycogen synthase kinase-3 (GSK3): regulation, actions, and diseases. Pharmacol Ther 148:114–131
Hooper C, Killick R, Lovestone S (2008) The GSK3 hypothesis of Alzheimer’s disease. J Neurochem 104:1433–1439
Jaworski T, Banach-Kasper E, Gralec K (2019) GSK-3β at the intersection of neuronal plasticity and neurodegeneration. Neural Plast 2019:4209475
Kaladchibachi SA, Doble B, Anthopoulos N, Woodgett JR, Manoukian AS (2007) Glycogen synthase kinase 3, circadian rhythms, and bipolar disorder: a molecular link in the therapeutic action of lithium. J Circadian Rhythms 5:3
Manoukian AS, Woodgett JR (2002) Role of glycogen synthase kinase-3 in cancer: regulation by Wnts and other signaling pathways. Adv Cancer Res 84:203–229
Gandy JC, Melendez-Ferro M, Bijur GN, Van Leuven F, Roche JK, Lechat B, Devijver H, Demedts D, Perez-Costas E, Roberts RC (2013) Glycogen synthase kinase-3β (GSK3β) expression in a mouse model of Alzheimer’s disease: a light and electron microscopy study. Synapse 67:313–327
Pandey GN, Dwivedi Y, Rizavi HS, Teppen T, Gaszner GL, Roberts RC, Conley RR (2009) GSK-3beta gene expression in human postmortem brain: regional distribution, effects of age and suicide. Neurochem Res 34:274–285
Takahashi M, Tomizawa K, Kato R, Sato K, Uchida T, Fujita SC, Imahori K (1994) Localization and developmental changes of tau protein kinase I/glycogen synthase kinase-3 beta in rat brain. J Neurochem 63:245–255
Leroy K, Brion JP (1999) Developmental expression and localization of glycogen synthase kinase-3beta in rat brain. J Chem Neuroanat 16:279–293
Pei JJ, Tanaka T, Tung YC, Braak E, Iqbal K, Grundke-Iqbal I (1997) Distribution, levels, and activity of glycogen synthase kinase-3 in the Alzheimer disease brain. J Neuropathol Exp Neurol 56:70–78
Hur EM, Zhou FQ (2010) GSK3 signalling in neural development. Nat Rev Neurosci 11:539–551
Zhang Y, Zhu C, Sun B, Lv J, Liu Z, Liu S, Li H (2017) Integrated high throughput analysis identifies GSK3 as a crucial determinant of p53-mediated apoptosis in lung cancer cells. Cell Physiol Biochem 42:1177–1191
Ucha M, Coria SM, Núñez AE, Santos-Toscano R, Roura-Martínez D, Fernández-Ruiz J, Higuera-Matas A, Ambrosio E (2019) Morphine self-administration alters the expression of translational machinery genes in the amygdala of male Lewis rats. J Psychopharmacol 33:882–893
Fatahi Z, Zeinaddini-Meymand A, Karimi-Haghighi S, Moradi M, Khodagholi F, Haghparast A (2020) Naloxone-precipitated withdrawal ameliorates impairment of cost-benefit decision making in morphine-treated rats: Involvement of BDNF, p-GSK3-β, and p-CREB in the amygdala. Neurobiol Learn Mem 167:107138
Eldar-Finkelman H (2002) Glycogen synthase kinase 3: an emerging therapeutic target. Trends Mol Med 8:126–132
Kitanaka N, Kitanaka J, Takemura M (2006) Modification of morphine-induced hyperlocomotion and antinociception in mice by clorgyline, a monoamine oxidase-A inhibitor. Neurochem Res 31:829–837
Martins DF, Rosa AO, Gadotti VM, Mazzardo-Martins L, Nascimento FP, Egea J, López MG, Santos AR (2011) The antinociceptive effects of AR-A014418, a selective inhibitor of glycogen synthase kinase-3 beta, in mice. J Pain 12:315–322
Wickens RH, Quartarone SE, Beninger RJ (2017) Inhibition of glycogen synthase kinase-3 by SB 216763 affects acquisition at lower doses than expression of amphetamine-conditioned place preference in rats. Behav Pharmacol 28:262–271
Kitanaka N, Kitanaka J, Takemura M (2003) Behavioral sensitization and alteration in monoamine metabolism in mice after single versus repeated methamphetamine administration. Eur J Pharmacol 474:63–70
Kitanaka J, Kitanaka N, Hall FS, Uhl GR, Tanaka K, Nishiyama N, Takemura M (2012) Straub tail reaction in mice treated with s1 receptor antagonist in combination with methamphetamine. Brain Res 1482:40–46
Tomita K, Yamanishi-Taira S, Igarashi K, Oogai Y, Kuwahara Y, Roudkenar MH, Roushandeh AM, Miyawaki S, Kurimasa A, Sato T (2022) Oxytocin ameliorates KCC2 decrease induced by oral bacteria-derived LPS that affect rat primary cultured cells and PC-12 cells. Peptides 150:170734
Cavalcante Morais T, Cavalcante Lopes S, Bezerra Carvalho KM, Rodrigues Arruda B, Correia de Souza FT, Salles Trevisan MT, Rao VS, Almeida Santos F (2012) Mangiferin, a natural xanthone, accelerates gastrointestinal transit in mice involving cholinergic mechanism. World J Gastroenterol 18:3207–3214
Porter-Stransky KA, Petko AK, Karne SL, Liles LC, Urs NM, Caron MG, Paladini CA, Weinshenker D (2020) Loss of β-arrestin2 in D2 cells alters neuronal excitability in the nucleus accumbens and behavioral responses to psychostimulants and opioids. Addict Biol 25:e12823
Cahill ME, Browne CJ, Wang J, Hamilton PJ, Dong Y, Nestler EJ (2018) Withdrawal from repeated morphine administration augments expression of the RhoA network in the nucleus accumbens to control synaptic structure. J Neurochem 147:84–98
Dajani R, Fraser E, Roe SM, Young N, Good V, Dale TC, Pearl LH (2001) Crystal structure of glycogen synthase kinase 3 beta: structural basis for phosphate-primed substrate specificity and autoinhibition. Cell 105:721–732
Hughes K, Nikolakaki E, Plyte SE, Totty NF, Woodgett JR (1993) Modulation of the glycogen synthase kinase-3 family by tyrosine phosphorylation. EMBO J 12:803–808
Wang QM, Fiol CJ, DePaoli-Roach AA, Roach PJ (1994) Glycogen synthase kinase-3 beta is a dual specificity kinase differentially regulated by tyrosine and serine/threonine phosphorylation. J Biol Chem 269:14566–14574
Del'Guidice T, Lemasson M, Beaulieu J-M (2011) Role of beta-Arrestin 2 downstream of dopamine receptors in the Basal Ganglia. Front Neuroanat 5
Shibasaki M, Masukawa D, Ishii K, Yamagishi Y, Mori T, Suzuki T (2013) Involvement of the K+–Cl− co-transporter KCC2 in the sensitization to morphine-induced hyperlocomotion under chronic treatment with zolpidem in the mesolimbic system. J Neurochem 125:747–755
Narita M, Suzuki T, Funada M, Misawa M, Nagase H (1993) Involvement of delta-opioid receptors in the effects of morphine on locomotor activity and the mesolimbic dopaminergic system in mice. Psychopharmacology 111:423–426
Barr JL, Unterwald EM (2020) Glycogen synthase kinase-3 signaling in cellular and behavioral responses to psychostimulant drugs. Biochim Biophys Acta Mol Cell Res 1867:118746
Horvitz JC (2002) Dopamine gating of glutamatergic sensorimotor and incentive motivational input signals to the striatum. Behav Brain Res 137:65–74
Laporte SA, Scott MGH (2019) beta-arrestins: multitask scaffolds orchestrating the where and when in cell signalling. Methods Mol Biol 1957:9–55
Bohn LM, Gainetdinov RR, Sotnikova TD, Medvedev IO, Lefkowitz RJ, Dykstra LA, Caron MG (2003) Enhanced rewarding properties of morphine, but not cocaine, in beta(arrestin)-2 knock-out mice. J Neurosci 23:10265–10273
Kitanaka J, Kitanaka N, Horie U, Kawasaki Y, Sakamoto T, Yashiro J, Tanaka K, Igarashi K, Tomita K, Shiomoto A, Watabe K, Nakano K, Takahashi H, Nishiyama N, Sato T, Takemura M (2021) Inhibitory effect of SB216763, a selective glycogen synthase kinase-3 inhibitor, on morphine-induced Straub’s tail reaction in mice. Proc Annu Meet Jpn Pharmacol Soc 94:3-P1–11
Funding
This study was supported, in part, by grants from Japan Society for the Promotion of Science KAKENHI (Grant Nos. 21K06591 to JK; 22K10151 to KT).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Junichi Kitanaka, Nobue Kitanaka, Kazuo Tomita, and F. Scott Hall. The first draft of the manuscript was written by Junichi Kitanaka, Nobue Kitanaka, Kazuo Tomita, and F. Scott Hall, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors confirm that this article content has no conflict of interest.
Ethics Approval
Animal handling and care were conducted in accordance with the Guide for the Care and Use of Laboratory Animals (8th edition, Institute of Laboratory Animal Resources-National Research Council, National Academy Press, 2011), and all experiments were reviewed and approved by the Institutional Animal Research Committee of Hyogo Medical University.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kitanaka, J., Kitanaka, N., Tomita, K. et al. Glycogen Synthase Kinase-3 Inhibitors Block Morphine-Induced Locomotor Activation, Straub Tail, and Depression of Rearing in Mice Via a Possible Central Action. Neurochem Res 48, 2230–2240 (2023). https://doi.org/10.1007/s11064-023-03902-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-023-03902-2