Abstract
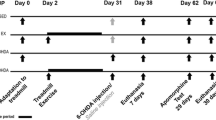
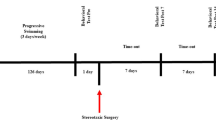
In the present study, we investigated the effects of physical exercise in the presence of Vitamin D3 (VD3), on 6-hydroxydopamine (6-OHDA)-lesioned hemiparkinsonian rats. The animals were divided into sham-operated (SO), 6-OHDA-lesioned, and 6-OHDA-lesioned plus VD3 (1 µg/kg, 21 days), in the absence (no exercise, NE) and presence (with exercise, WE) of physical exercise on a treadmill (30 min, speed of 20 cm/s, once a day/21 days). This procedure started, 24 h after the stereotaxic surgery (injections of 6-OHDA into the right striatum). The animals were then subjected to behavioral (rotarod, open field, and apomorphine tests) and their brain areas were dissected for neurochemical, dopamine (DA), 3,4-dihydroxyphenylacetic acid (DOPAC) determinations, and immunohistochemical studies for tyrosine hydroxylase (TH), dopamine transporter (DAT), and vitamin D receptor (VD3R). The effects on the brain oxidative stress: nitrite/nitrate, glutathione (GSH), and malondialdehyde (MDA) measurements were also evaluated. Behavioral changes of the 6-OHDA lesioned group were improved by exercise plus VD3. Similar results were observed in dopamine (DA) and 3,4-dihydroxyphenylacetic acid (DOPAC) concentrations increased by exercise and VD3, compared with SO groups. Additionally, tyrosine hydroxylase (TH) and dopamine transporter (DAT) immunoexpressions were decreased in the 6-OHDA-lesioned groups, with values normalized after exercise and VD3. The VD3 receptor immunoexpression decreased in the 6-OHDA (NE) group, and this was attenuated by exercise, especially after VD3. While 6-OHDA lesions increased, VD3 supplementation decreased the oxidative stress, which was intensified by exercise. VD3 showed neuroprotective properties that were intensified by physical exercise. These VD3 actions on hemiparkinsonian rats are possibly related to its antioxidant and anti-inflammatory effects.










Similar content being viewed by others
Data Availability
All data generated or analyzed during this study are included in this manuscript. Further inquiries can be directed and are available upon request to the corresponding author.
References
Evatt ML, Delong MR, Khazai N, Rosen A, Triche S, Tangpricha V (2008) Prevalence of vitamin d insufficiency in patients with Parkinson’s disease and Alzheimer’s disease. Arch Neurol 65(10):1348–1352. https://doi.org/10.1001/archneur.65.10.1348
Knekt P, Killkkinen A, Rissanen H, Marniemi J, Saaksjarvi K, Heliovaara M (2010) Serum vitamin D and the risk of Parkinson’s disease. Arch Neurol 67:808–811
Luo X, Ou R, Dutta R, Tian Y, Xiong H, Shang H (2018) Association between serum vitamin D levels and Parkinson’s disease: a systematic review and meta-analysis. Front Neurol. https://doi.org/10.3388/fneur.2018.00909
Fullard ME, Duda JE (2020) A review of the relationship between vitamin D and Parkinson’s disease symptoms. Front Neurol. https://doi.org/10.3389/fneur.2020.00454
Mak MKY, Wong-Yu ISK (2019) Exercise for Parkinson’s disease. Int Rev Neurobiol 147:1–44
Schootemeijer S, van der Kolk NM, Bloem BR, de Vries NM (2020) Current perspectives on aerobic exercise in people with Parkinson’s disease. Neurotherapeutics 17:1418–1433
Carvalho AO, Sá-Filho AS, Murillo-Rodriguez E, Rocha NB, Carta MG, Machado S (2018) Physical exercise for Parkinson’s disease: clinical and experimental evidence. Clin Practi Epidemiol Ment Health 14:89–98
Bhalsing KS, Abbas MM, Tan LCS (2018) Role of physical activity in Parkinson’s disease ann Indian. Acad Neurol 21(4):242–249
Costa RO, Gadelha-Filho CVJ, Costa AEM, Feitosa ML, Araujo DP, Lucena JD, de Aquino PEA, Lima FAV, Neves KRT, Viana GSB (2017) The treadmill exercise protects against dopaminergic neuron loss and brain oxidative stress in parkinsonian rats. Oxid Med Cell Longev 2017:2138169
Conceição LR, Moura LP, Pauli JR (2019) Benefits of physical exercise on Parkinson’s disease disorders induced in animal models. Motriz. https://doi.org/10.1590/S1980-6574201900030007
Shen X, Wong-Yu IS, Mak MK (2016) Effects of exercise on falls, balance, and gait ability in Parkinson’s disease: a meta-analysis. Neurorehabil Neural Repair 30(6):512–527
Kalyani RR, Stein B, Valiyil R, Manno R, Maynard JW, Crews D (2010) Vitamin D treatment for the prevention of falls in older adults: systematic review and meta-analysis. J Am Geriatr Soc 58:1299–1310
Imaoka M, Higuchi Y, Todo E, Kitagawa T, Ueda T (2016) Low-frequency exercise and vitamin D supplementation reduces falls among institutionalized frail elderly. Int J Gerontol 10:202–206
Antoniak AE, Greig CA (2019) The effect of combined resistance exercise training and vitamin D3 supplementation on musculoskeletal health and function in older adults: a systematic review and meta-analysis. BMJ Open. https://doi.org/10.1136/bmjopen-2016-014619
Sleeman J, Aspray T, Lawson R, Coleman S, Duncan G, Khoo TK, Schoenmakers I, Rochester L, Burn D, Yarnall A (2017) The role of vitamin D in disease progression in early Parkinson’s disease. J Parkinson’s Dis 7:669–675
Canning CG, Sherrington C, Lord SR, Close JC, Heritier S, Heller GZ, Howard K, Allen NE, Latt MD, Murray SM, O’Rourke SD, Paul SS, Song J, Fung VS (2015) Exercise for falls prevention in Parkinson disease: a randomized controlled trial. Neurology 84(3):304–312
Annweiler C, Montero-Odasso M, Schott AM, Berrut G, Fantino B, Beauchet O (2010) Fall prevention and vitamin D in the elderly: an overview of the key role of the non-bone effects. J Neuroeng Rehabil 7:50. https://doi.org/10.1186/1743-0003-7-50
Zhang HJ, Zhang JR, Mao CJ, Li K, Wang F, Chen J, Liu CF (2019) Relationship between 25-Hydroxyvitamin D, bone density, and Parkinson’s disease symptoms. Acta Neurol Scand 140(4):274–280. https://doi.org/10.1111/ane.13141
Barichella M, Garrì F, Caronni S, Bolliri C, Zocchi L, Macchione MC, Ferri V, Calandrella D, Pezzoli G (2022) Vitamin D status and Parkinson’s disease. Brain Sci 12(6):790. https://doi.org/10.3390/brainsci12060790
Pignolo A, Mastrilli S, Davì C, Arnao V, Aridon P, dos Santos Mendes FA, Gagliardo C, D’Amelio M (2022) Vitamin D and Parkinson’s disease. Nutrients 14:1220. https://doi.org/10.3390/nu14061220
Luong KVQ, Nguyên LTH (2017) Vitamin D and Parkinson’s disease. J Neurosci Res 90:2227–2236
Luthra NS, Kim S, Zhang Y, Christine CW (2018) Characterization of vitamin D supplementation and clinical outcomes in a large cohort of early Parkinson’s disease. J Clin Mov Disord 5:7. https://doi.org/10.1186/s40734-018-0074-6
Lima LAR, Lopes MJP, Costa RO, Lima FAV, Neves KRT, Andrade GM, Viana GSB (2018) Vitamin D protects dopaminergic neurons against neuroinflammation and oxidative stress in hemiparkinsonian rats. J Neuroinflamm 15:249. https://doi.org/10.1186/s12974-018-1266-6
Liu Z, Zhou T, Ziegler AC, Dimitrion P, Zuo L (2013) Oxidative stress in neurodegenerative diseases: from molecular mechanisms to clinical applications. J Exerc Rehabil 9:354–361
Leal LKAM, Lima LA, Aquino PEA, Sousa JAC, Gadelha-Filho CVJ, Calou IBF, Lopes MJP, Lima FAV, Neves KRT, Andrade GM, Viana GSB (2020) Vitamin D (VD3) antioxidative and anti-inflammatory activities: peripheral and central effects. Eur J Pharmacol 879:173099
Hald A, Lotharius J (2016) Oxidative stress and inflammation in Parkinson’s disease: is there a causal link? J Parkinson’s Dis 6(1):29–37
Tillerson JL, Caudle WM, Reverón ME, Miller GW (2003) Exercise induces behavioral recovery and attenuates neurochemical deficits in rodent models of Parkinson’s disease. Neuroscience 119:899–911
Chen YH, Kuo TT, Kao JH, Huang EY, Hsieh TH, Chou YC, Hoffer BJ (2018) Exercise ameliorates motor deficits and improves dopaminergic functions in the rat hemi-Parkinson’s model. Sci Rep 8:3973
Iancu R, Mohapel P, Brundin P, Paul G (2005) Behavioral characterization of a unilateral 6-OHDA-lesion model of Parkinson’s disease in mice. Behav Brain Res 162:1–10
Su RJ, Zhen JL, Wang W, Zhan JL, Zheng Y, Wang XM (2017) Time-course behavioral features are correlated with Parkinson’s disease-associated pathology in a 6-hydroxydopamine hemiparkinsonian rat model. Mol Med Rep. https://doi.org/10.3892/mmr.2017.8277
Carter RJ, Morton J, Dunnett SB (2001) Motor coordination and balance in rodents. Curr Protoc Neurosci. https://doi.org/10.1002/0471142301.ns0812s15
Hamm RJ, Pike BR, O’Dell DM, Lyeth BG, Jenkins LW (1994) The rotarod test: an evaluation of its effectiveness in assessing motor deficits following traumatic brain injury. J Neurotrauma 11(2):187–196
Riedesel AK, Helgers SOA, Abdulbaki A, Hatipoglu Majernik G, Alam M, Krauss JK, Schwabe K (2021) Severity assessment of complex and repeated intracranial surgery in rats. Eur Surg Res. https://doi.org/10.1159/000520678
Seibenhener ML, Wooten MC (2015) Use of the open field maze to measure locomotor and anxiety-like behavior in mice. J Vis Exp 96:52434. https://doi.org/10.3791/52434
Johnson ME, Salvatore MF, Maiolo SA, Bobrovskaya L (2018) Tyrosine hydroxylase as a sentinel for central and peripheral tissue responses in Parkinson’s progression: evidence from clinical studies and neurotoxin models. Prog Neurobiol 165–167:1–25
Daubner SC, Le T, Wang S (2011) Tyrosine hydroxylase and regulation of dopamine synthesis. Arch BiochemBiophys 508:1–12
Vaughan RA, Foster JD (2013) Mechanisms of dopamine transporter regulation in normal and disease states. Trends Pharmacol Sci. https://doi.org/10.1016/j.tips.2013.07.005
Shih MC, Hoexter MQ, Andrade LA, Bressan RA (2006) Parkinson’s disease and dopamine transporter neuroimaging: a critical review. Sao Paulo Med J 124(3):168–175
Butler MW, Burt A, Edwards TL, Zuchner S, Scott WK, Martin ER, Vance JM, Wang L (2011) Vitamin D receptor gene as a candidate gene for Parkinson’s disease. Am Hum Genet 75:201–210
de Aquino PEA, Lustosa IR, Sousa SNS, Chaves-Filho AJM, Lima FAV, Santos ADC, Gramosa NV, Silveira ER, Viana GSB (2020) The N-Methyl-(2S, 4R)-trans-4-hydroxy-L-proline-enriched methanol fraction from Sideroxylon obtusifolium shows an anticonvulsant activity associated with its anti-inflammatory/ antioxidant actions. Planta Med Int Open. 7:e158–e169
Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR (1982) Analysis of nitrate, nitrite, and nitrate in biological fluids. Anal Biochem 126:131–138
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95(2):351–358. https://doi.org/10.1016/0003-2697(79)90738-3
Draper HH, Hadley M (1990) Malondialdehyde determination as index of lipid peroxidation. Methods Enzymol 186:421–431
Sedlák J, Hanus L (1982) Changes of glutathione and protein-bound SH-groups concentration in rat adrenals under acute and repeated stress. Endocrinol Exp 16(2):103–109
Borrione P, Tranchita E, Sansone P, Parisi A (2014) Effects of physical activity in Parkinson’s disease: a new tool for rehabilitation. World J Methodol 26:133–143
Shu H-F, Yang T, Yu S-X, Huang H-D, Jiang L-L, Gu J-W, Kuang Y-Q (2014) Aerobic exercise for Parkinson’s disease: a systematic review and meta-analysis of randomized controlled trials. PLoS ONE 9(7):e100503
Murray DK, Sacheli MA, Eng JJ, Stoessl AJ (2014) The effects of exercise on cognition in Parkinson’s disease: a systematic review. Transl Neurodegener 3(1):5
Hou L, Chen W, Liu X, Qiao D, Zhou F-M (2017) Exercise-induced neuroprotection of the nigrostriatal dopamine system in Parkinson’s disease. Front Aging Neurosci. https://doi.org/10.3380/fnagi.2017.00358
Ma C-L, Ma X-T, Wang J-J, Liu H, Chen Y-F, Yang Y (2017) Physical exercise induces hippocampal neurogenesis and prevents cognitive decline. Behav Brain Res 317:332–339
Guillot X, Semerano L, Saidenberg-Kermanac’h N, Falgarone G, Boissier MC (2010) Vitamin D and inflammation. Joint Bone Spine 77:552–557
Carnnell JJ, Grant WB, Holick MF (2014) Vitamin D and inflammation. Dermato-Endocrinology 61:e983401
Mousa A, Misso M, Teede H, Scragg R, Courten B (2016) Effect of Vitamin D supplementation on inflammation: protocol for a systematic review. BMJ Open 6:e010804. https://doi.org/10.1136/bmjopen-2015-010804
Guzman-Martinez L, Maccioni RB, Andrade V, Navarrete LP, Pastor MG, Ramos-Escobar N (2019) Neuroinflammation as a common feature of neurodegenerative disorders. Front Pharmacol 10:1008
Kempuraj D, Thangavel R, Selvakumar GP, Zaheer S, Ahmed ME, Raikwar SP, Iyer S, Bhagavan SM, Beladakere-Ramaswamy S, Zaheer A (2017) Brain, and peripheral atypical inflammatory mediators potentiate neuroinflammation and neurodegeneration. Front Cell Neurosci. https://doi.org/10.3389/fncel.2017.00216
Petzinger GM, Holschneider DP, Fischer BE, McEwen S, Kintz N, Halliday M, Toy W, Walsh JW, Beeler J, Jakowec MW (2015) The effects of exercise on dopamine neurotransmission in Parkinson’s disease: targeting neuroplasticity to modulate basal ganglia circuitry. Brain Plast 1:29–39
Sacheli MA, Neva JL, Lakhani B, Murray DK, Vafai N, Shahinfard E, English C, McCormick S, Dinelle K, Neilson N, McKenzie J, Schulzer M, McKenzie DC, Appel-Cresswell S, McKeown MJ, Boyd LA, Sossi V, Stoessl AJ (2019) Exercise increases caudate dopamine release and ventral striatal activation in Parkinson’s disease. Mov Disord 34:1891–1900
Palasz E, Niewiadomski W, Gasiorowska A, Wysocka A, Stepniewska A, Niewiadomska G (2019) Exercise-induced neuroprotection and recovery of motor function in animal models of Parkinson’s disease. Front Neurol. https://doi.org/10.3389/fneur.2019.01143
Haavick J, Toska K (1998) Tyrosine hydroxylase and Parkinson’s disease. Mol Neurobiol 16:285–309
Tabrez S, Jabir NR, Shakil S, Greig NH, Alam Q, Abuzenadah AM, Damanhouri GA, Kamal MA (2012) A synopsis on the role of tyrosine hydroxylase in Parkinson’s disease. CNS Neurol Disord Drug Targets 11:395–409
Chen Y, Lian Y, Ma Y, Wu C, Zheng Y, Xie N (2017) The expression and significance of tyrosine hydroxylase in the brain tissue of Parkinson’s’ disease rats. Exp Ther Med 14(5):4813–4816
Orme RP, Bhangal MS, Fricker RA (2013) Calcitriol imparts neuroprotection in vitro to midbrain dopaminergic neurons by upregulating GDNF expression. PLoS ONE 8(4):e62040
Cui X, Pertile R, Liu P, Eyles DW (2015) Vitamin D regulates tyrosine hydroxylase expression: N-cadherin is a possible mediator. Neurosci 304:90–100
Nutt JG, Carter JH, Sexton GJ (2004) The dopamine transporter: importance in Parkinson’s disease. Ann Neurol 55:766–773
Ikeda K, Ebina J, Kawabe K, Iwasaki Y (2019) Dopamine transporter imaging in Parkinson disease: progressive changes and therapeutic modification after anti-parkinsonian medications. Intern Med 58:1665–1672
Dias V, Junn E, Mouradian MM (2013) The role of oxidative stress in Parkinson’s disease. J Parkinsons Dis 3(4):461–491
Araújo de Lima L, Oliveira Cunha PL, Felicio Calou IB, Tavares Neves KR, Facundo HT, de Barros Viana GS (2022) Effects of vitamin D (VD3) supplementation on the brain mitochondrial function of male rats, in the 6-OHDA-induced model of Parkinson’s disease. Neurochem Int. https://doi.org/10.1016/j.neuint.2022.105280
Park JS, Davis RL, Sue CM (2018) Mitochondrial dysfunction in Parkinson’s disease: new mechanistic insights and therapeutic perspectives. Curr Neurol Neurosci Rep 18(5):21. https://doi.org/10.1007/s11910-018-0829-3
Wright R (2022) Mitochondrial dysfunction and Parkinson’s disease. Nat Neurosci 25:2. https://doi.org/10.1038/s41593-021-00989-0NEURODEGENERATIVEDISEASE
Bayo-Olugbami A, Nafiu AB, Amin A, Ogundele OM, Lee CC, Owoyele BV (2022) Vitamin D attenuated 6-OHDA-induced behavioural deficits, dopamine dysmetabolism, oxidative stress, and neuro-inflammation in mice. Nutr Neurosci 25(4):823–834
Pike JW, Meyer MB (2010) The vitamin D receptor: new paradigms for the regulation of gene expression by 1,25-dihydroxyvitamin D3. Endocrinol Metab Clin North Am 39:255–269
Hirsch EC, Hunot S (2009) Neuroinflammation in Parkinson’s disease: a target for neuroprotection? Lancet Neurol 8:382–397
Hirsch EC, Vyas S, Hunot S (2012) Neuroinflammation in Parkinson’s disease. Parkinsonism Relat Disord 18(Suppl 1):S210–S212
Jenner P (2003) Oxidative stress in Parkinson’s disease. Ann Neurol 53(Suppl 3):S26-36
Blesa J, Trigo-Damas I, Quiroga-Varela A, Jackson-Lewis VR (2015) Oxidative stress and Parkinson’s disease. Front Neuroanat 9:91
Chang K-H, Chen C-M (2020) The role of oxidative stress in Parkinson’s disease. Antioxidants 9(7):597
Kim GH, Kim JE, Rhie SJ, Yoon S (2015) The role of oxidative stress in neurodegenerative diseases. Exp Neurobiol 24(4):325–340
Wei Z, Li X, Li X, Liu Q, Cheng Y (2018) Oxidative stress in Parkinson’s disease: a systematic review and meta-analysis. Front Mol Neurosci 5(11):236
Martin HL, Teismann P (2009) Glutathione-a review on its role and significance in Parkinson’s disease. FASEB J 23(10):3263–3272
Bjørklund G, Peana M, Maes M, Dadar M, Severin B (2021) The glutathione system in Parkinson’s disease and its progression. Neurosci Biobehav Ver 120:470–478
Aoyama K, Nakaki T (2013) Impaired glutathione synthesis in neurodegeneration. Int J Mol Sci 14(10):21021–21044
Aoyama K (2021) Glutathione in the brain. Int J Mol Sci 22(9):5010
Iskusnykh IY, Zakharova AA, Pathak D (2022) Glutathione in brain disorders and aging. Molecules 27:324
Wang HL, Zhang J, Li YP, Dong L, Chen YZ (2021) Potential use of glutathione as a treatment for Parkinson’s disease. Exp Ther Med 21(2):125
Crotty GF, Schwarzschild MA (2020) Chasing protection in Parkinson’s disease: does exercise reduce risk and progression? Front Aging Neurosci 12:186
Lv L, Tan X, Peng X, Bai R, Xiao Q, Zou T, Tan J, Zhang H, Wang C (2020) The relationships of vitamin D, vitamin D receptor gene polymorphisms, and vitamin D supplementation with Parkinson’s disease. Transl Neurodegener 9(1):34
Xu X, Fu Z, Le W (2019) Exercise and Parkinson’s disease. Int Rev Neurobiol 147:45–74
Tsukita K, Sakamaki-Tsukita H, Takahashi R (2022) Long-term effect of regular physical activity and exercise habits in patients with early Parkinson disease. Neurology 98(8):e859–e871
McKenna C, Salvador A, Askow A, Paulussen KJM, Keeble A, Paluska S, De Lisio M, Khan N, Burd N (2021) Higher protein intake does not potentiate skeletal muscle vitamin D receptor. Curr Dev Nutr 5(Supplement_2):512
Lithgow H, Florida-James G, Ross M, Duncan G, Leggate M (2021) Exercise acutely increases vitamin D receptor expression in T lymphocytes in vitamin D-deficient men, independent of age. Exp Physiol 106(7):1460–1469. https://doi.org/10.1113/EP089480
Latham CM, Brightwell CR, Keeble AR, Munson BD, Thomas NT, Zagzoog AM, Fry CS, Fry JL (2021) Vitamin D promotes skeletal muscle regeneration and mitochondrial health. Front Physiol 14(12):660498. https://doi.org/10.3389/fphys.2021.660498.PMID:33935807;PMCID:PMC8079814
Latimer CS, Brewer LD, Searcy JL, Chen KC, Popović J, Kraner SD, Thibault O, Blalock EM, Landfield PW, Porter NM (2014) Vitamin D prevents cognitive decline and enhances hippocampal synaptic function in aging rats. Proc Natl Acad Sci USA 111(41):E4359–E4366. https://doi.org/10.1073/pnas.1404477111
Redenšek S, Kristanc T, Blagus T, Trošt M, Dolžan V (2022) Genetic variability of the vitamin D receptor affects susceptibility to Parkinson’s disease and dopaminergic treatment adverse events. Front Aging Neurosci 19(14):853277. https://doi.org/10.3389/fnagi.2022.853277
Acknowledgements
The authors are grateful to Prof. Francisco Vagnaldo Fechine Jamacuru and Prof. Héber José de Moura for their expertise in statistical data analyses, and also to Prof. M.O.L. Viana for the grammatical orthographic revision of the manuscript.
Funding
The authors are grateful for the financial support of the Brazilian National Research Council (CNPq), the Coordination for Improvement of Higher Level Personnel (CAPES), and the Foundation for Scientific and Technological Development Support of the State of Ceará (FUNCAP).
Author information
Authors and Affiliations
Contributions
ROdC: Conducted all behavioral tests; CVJGF: Helped in handling and caring for the animals’ maintenance; PEAdA and LARL and JDdL: Carried out all the biochemical assays; WLCR: Responsible for the animal’s care and handling; FAVL and KRTN: Carried out all the immunohistochemical assays; GSdBV: Coordinated the project and wrote the final version of the manuscript, read and approved by all authors.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Approval
The research followed the ethical principles of the Brazilian National Council for Animal Experimentation (CONCEA). The study was submitted to and approved by the Animal Research Ethics Committee (CEPA) of the Faculty of Medicine of the Federal University of Ceará, under protocol number 82/2016. The experiments were performed according to the Guide for the Care and Use of Laboratory Animals: Eighth Edition, National Academy of Sciences, Institute for Laboratory Animal Research (ILAR), in 2011. NIH (Office of Laboratory Animal Welfare).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
da Costa, R.O., Gadelha-Filho, C.V.J., de Aquino, P.E.A. et al. Vitamin D (VD3) Intensifies the Effects of Exercise and Prevents Alterations of Behavior, Brain Oxidative Stress, and Neuroinflammation, in Hemiparkinsonian Rats. Neurochem Res 48, 142–160 (2023). https://doi.org/10.1007/s11064-022-03728-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-022-03728-4