Abstract
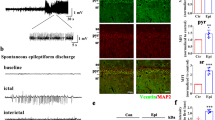
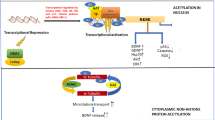
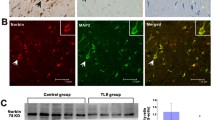
Recent studies have shown that histone acetylation is involved with the regulation of enzyme glutamate decarboxylases (GADs), including GAD67 and GAD65. Here, we investigated the histone acetylation modifications of GADs in the pathogenesis of epilepsy and explored the therapeutic effect of a novel second-generation histone deacetylase inhibitor (HDACi) JNJ-26481585 in epilepsy animals. We revealed the suppression of GADs protein and mRNA level, and histone hypoacetylation in patients with temporal lobe epilepsy and pilocarpine-induced epilepsy mice model. Double-immunofluorescence also indicated that the hypoacetyl-H3 was located in hippocampal GAD67/GAD65 positive neurons in epilepsy mice. JNJ-26481585 significantly reversed the decrease of the GAD67/GAD65 both protein and mRNA levels, and the histone hypoacetylation of GABAergic neurons in epilepsy mice. Meanwhile, single-cell real-time PCR performed in GFP-GAD67/GAD65 transgenic mice demonstrated that JNJ-26481585 induced increase of GAD67/GAD65 mRNA level in GABAergic neurons. Furthermore, JNJ-26481585 significantly alleviated the epileptic seizures in mice model. Together, our findings demonstrate inhibition of GADs gene via histone acetylation plays an important role in the pathgenesis of epilepsy, and suggest JNJ-26481585 as a promising therapeutic strategy for epilepsy.





Similar content being viewed by others
References
Dulac O, Milh M, Holmes GL (2013) Brain maturation and epilepsy. Handb Clin Neurol 111:441–446
Holmes GL, Milh MD, Dulac O (2012) Maturation of the human brain and epilepsy. Handb Clin Neurol 107:135–143
Mizielinska S, Greenwood S, Connolly CN (2006) The role of GABAA receptor biogenesis, structure and function in epilepsy. Biochem Soc Trans 34:863–867
Jagirdar R, Drexel M, Kirchmair E, Tasan RO, Sperk G (2015) Rapid changes in expression of class I and IV histone deacetylases during epileptogenesis in mouse models of temporal lobe epilepsy. Exp Neurol 273:92–104
Lusardi TA, Akula KK, Coffman SQ, Ruskin DN, Masino SA, Boison D (2015) Ketogenic diet prevents epileptogenesis and disease progression in adult mice and rats. Neuropharmacology 99:500–509
Ritter C, Gobel CH, Liebig T, Kaminksy E, Fink GR, Lehmann HC (2015) An epigenetic cause of seizures and brain calcification: pseudohypoparathyroidism. Lancet 385:1802
Parrish RR, Buckingham SC, Mascia KL, Johnson JJ, Matyjasik MM, Lockhart RM, Lubin FD (2015) Methionine increases BDNF DNA methylation and improves memory in epilepsy. Ann Clin Transl Neurol 2:401–416
Miller-Delaney SF, Bryan K, Das S, McKiernan RC, Bray IM, Reynolds JP, Gwinn R, Stallings RL, Henshall DC (2015) Differential DNA methylation profiles of coding and non-coding genes define hippocampal sclerosis in human temporal lobe epilepsy. Brain J Neurol 138:616–631
Kobow K, Blumcke I (2014) Epigenetic mechanisms in epilepsy. Prog Brain Res 213:279–316
Hwang JY, Aromolaran KA, Zukin RS (2013) Epigenetic mechanisms in stroke and epilepsy. Neuropsychopharmacol Off Publ Am Coll Neuropsychopharmacol 38:167–182
Roopra A, Dingledine R, Hsieh J (2012) Epigenetics and epilepsy. Epilepsia 53(Suppl 9):2–10
Belhedi N, Perroud N, Karege F, Vessaz M, Malafosse A, Salzmann A (2014) Increased CPA6 promoter methylation in focal epilepsy and in febrile seizures. Epilepsy Res 108:144–148
Fukuchi M, Nii T, Ishimaru N, Minamino A, Hara D, Takasaki I, Tabuchi A, Tsuda M (2009) Valproic acid induces up- or down-regulation of gene expression responsible for the neuronal excitation and inhibition in rat cortical neurons through its epigenetic actions. Neurosci Res 65:35–43
Zhang Z, Cai YQ, Zou F, Bie B, Pan ZZ (2011) Epigenetic suppression of GAD65 expression mediates persistent pain. Nat Med 17:1448–1455
Banerjee K, Akiba Y, Baker H, Cave JW (2013) Epigenetic control of neurotransmitter expression in olfactory bulb interneurons. Int J Dev Neurosci Off J Int Soc Dev Neurosci 31:415–423
Ueki N, Lee S, Sampson NS, Hayman MJ (2013) Selective cancer targeting with prodrugs activated by histone deacetylases and a tumour-associated protease. Nat Commun 4:2735
Benson MJ, Manzanero S, Borges K (2015) Complex alterations in microglial M1/M2 markers during the development of epilepsy in two mouse models. Epilepsia 56:895–905
Oliveira CV, Grigoletto J, Funck VR, Ribeiro LR, Royes LF, Fighera MR, Furian AF, Oliveira MS (2015) Evaluation of potential gender-related differences in behavioral and cognitive alterations following pilocarpine-induced status epilepticus in C57BL/6 mice. Physiol Behav 143:142–150
Leung A, Ahn S, Savvidis G, Kim Y, Iskandar D, Luna MJ, Kim KS, Cunningham M, Chung S (2015) Optimization of pilocarpine-mediated seizure induction in immunodeficient NodScid mice. Epilepsy Res 109:114–118
Castillo CG, Mendoza S, Freed WJ, Giordano M (2006) Intranigral transplants of immortalized GABAergic cells decrease the expression of kainic acid-induced seizures in the rat. Behav Brain Res 171:109–115
Racine RJ (1972) Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalogr Clin Neurophysiol 32:281–294
Racine R, Okujava V, Chipashvili S (1972) Modification of seizure activity by electrical stimulation. 3 Mechanisms. Electroencephalogr Clin Neurophysiol 32:295–299
Fang M, Shen L, Yin H, Pan YM, Wang L, Chen D, Xi ZQ, Xiao Z, Wang XF, Zhou SN (2011) Downregulation of gephyrin in temporal lobe epilepsy neurons in humans and a rat model. Synapse 65:1006–1014
Kash SF, Johnson RS, Tecott LH, Noebels JL, Mayfield RD, Hanahan D, Baekkeskov S (1997) Epilepsy in mice deficient in the 65-kDa isoform of glutamic acid decarboxylase. Proc Natl Acad Sci USA 94:14060–14065
Mathew J, Balakrishnan S, Antony S, Abraham PM, Paulose CS (2012) Decreased GABA receptor in the cerebral cortex of epileptic rats: effect of Bacopa monnieri and Bacoside-A. J Biomed Sci 19:25
Sakakibara T, Sukigara S, Otsuki T, Takahashi A, Kaneko Y, Kaido T, Saito Y, Sato N, Nakagawa E, Sugai K, Sasaki M, Goto Y, Itoh M (2012) Imbalance of interneuron distribution between neocortex and basal ganglia: consideration of epileptogenesis of focal cortical dysplasia. J Neurol Sci 323:128–133
Darrah SD, Miller MA, Ren D, Hoh NZ, Scanlon JM, Conley YP, Wagner AK (2013) Genetic variability in glutamic acid decarboxylase genes: associations with post-traumatic seizures after severe TBI. Epilepsy Res 103:180–194
Malter MP, Frisch C, Zeitler H, Surges R, Urbach H, Helmstaedter C, Elger CE, Bien CG (2015) Treatment of immune-mediated temporal lobe epilepsy with GAD antibodies. Seizure 30:57–63
He S, Bausch SB (2014) Synaptic plasticity in glutamatergic and GABAergic neurotransmission following chronic memantine treatment in an in vitro model of limbic epileptogenesis. Neuropharmacology 77:379–386
Alvestad S, Hammer J, Qu H, Haberg A, Ottersen OP, Sonnewald U (2011) Reduced astrocytic contribution to the turnover of glutamate, glutamine, and GABA characterizes the latent phase in the kainate model of temporal lobe epilepsy. J Cereb Blood Flow Metab Off J Int Soc Cereb Blood Flow Metab 31:1675–1686
Rajasekaran K, Kapur J, Bertram EH (2007) Alterations in GABA(A) receptor mediated inhibition in adjacent dorsal midline thalamic nuclei in a rat model of chronic limbic epilepsy. J Neurophysiol 98:2501–2508
Austin JE, Buckmaster PS (2004) Recurrent excitation of granule cells with basal dendrites and low interneuron density and inhibitory postsynaptic current frequency in the dentate gyrus of macaque monkeys. J Comp Neurol 476:205–218
Kobayashi M, Buckmaster PS (2003) Reduced inhibition of dentate granule cells in a model of temporal lobe epilepsy. J Neurosci Off J Soc Neurosci 23:2440–2452
Georgieva Z, Parton M (2014) Cerebellar ataxia and epilepsy with anti-GAD antibodies treatment with IVIG and plasmapheresis. BMJ Case Rep 2014:bcr2013202314
Leke R, Silveira TR, Escobar TD, Schousboe A (2014) Expression of Glutamate Decarboxylase (GAD) mRNA in the brain of bile duct ligated rats serving as a model of hepatic encephalopathy. Neurochem Res 39:605–611
Nasreen Z, Jameel T, Hasan A, Parveen N, Sadasivudu B (2012) Glutamate decarboxylase and GABA aminotransferase levels in different regions of rat brain on the onset of Leptazol induced convulsions. Neurochem Res 37:202–204
Erlander MG, Tillakaratne NJ, Feldblum S, Patel N, Tobin AJ (1991) Two genes encode distinct glutamate decarboxylases. Neuron 7:91–100
Esclapez M, Tillakaratne NJ, Kaufman DL, Tobin AJ, Houser CR (1994) Comparative localization of two forms of glutamic acid decarboxylase and their mRNAs in rat brain supports the concept of functional differences between the forms. J Neurosci Off J Soc Neurosci 14:1834–1855
Shen X, Liu Y, Xu S, Zhao Q, Wu H, Guo X, Shen R, Wang F (2014) Menin regulates spinal glutamate-GABA balance through GAD65 contributing to neuropathic pain. Pharmacol Rep PR 66:49–55
Kundakovic M, Chen Y, Guidotti A, Grayson DR (2009) The reelin and GAD67 promoters are activated by epigenetic drugs that facilitate the disruption of local repressor complexes. Mol Pharmacol 75:342–354
Krumholz A, Wiebe S, Gronseth G, Shinnar S, Levisohn P, Ting T, Hopp J, Shafer P, Morris H, Seiden L, Barkley G, French J, Quality Standards Subcommittee of the American Academy of N, American Epilepsy S (2007) Practice parameter: evaluating an apparent unprovoked first seizure in adults (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology and the American Epilepsy Society. Neurology 69:1996–2007
Stuhmer T, Arts J, Chatterjee M, Borawski J, Wolff A, King P, Einsele H, Leo E, Bargou RC (2010) Preclinical anti-myeloma activity of the novel HDAC-inhibitor JNJ-26481585. Br J Haematol 149:529–536
Capasso KE, Manners MT, Quershi RA, Tian Y, Gao R, Hu H, Barrett JE, Sacan A, Ajit SK (2015) Effect of histone deacetylase inhibitor JNJ-26481585 in pain. J Mol Neurosci MN 55:570–578
Arts J, King P, Marien A, Floren W, Belien A, Janssen L, Pilatte I, Roux B, Decrane L, Gilissen R, Hickson I, Vreys V, Cox E, Bol K, Talloen W, Goris I, Andries L, Du Jardin M, Janicot M, Page M, van Emelen K, Angibaud P (2009) JNJ-26481585, a novel “second-generation” oral histone deacetylase inhibitor, shows broad-spectrum preclinical antitumoral activity. Clin Cancer Res Off J Am Assoc Cancer Res 15:6841–6851
Acknowledgments
This study was supported by Grants of the National Natural Science Foundation of China (Nos. 81201005, 81501115).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Jin-Gang Wang, Qing Cai and Jun Zheng have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Wang, JG., Cai, Q., Zheng, J. et al. Epigenetic Suppression of GADs Expression is Involved in Temporal Lobe Epilepsy and Pilocarpine-Induced Mice Epilepsy. Neurochem Res 41, 1751–1760 (2016). https://doi.org/10.1007/s11064-016-1891-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-016-1891-3