Abstract
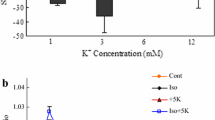
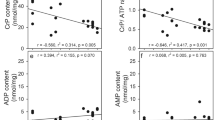
The importance of astrocytic K+ uptake for extracellular K+ ([K+]e) clearance during neuronal stimulation or pathophysiological conditions is increasingly acknowledged. It occurs by preferential stimulation of the astrocytic Na+,K+-ATPase, which has higher Km and Vmax values than its neuronal counterpart, at more highly increased [K+]e with additional support of the cotransporter NKCC1. Triggered by a recent DiNuzzo et al. paper, we used administration of the glycogenolysis inhibitor DAB to primary cultures of mouse astrocytes to determine whether K+ uptake required K+-stimulated glycogenolysis. KCl was increased by either 5 mM (stimulating only the Na+,K+-ATPase) or 10 mM (stimulating both transporters) in glucose-containing saline media prepared to become iso-osmotic after the addition. DAB completely inhibited both uptakes, the Na+,K+-ATPase-mediated by preventing Na+ uptake for stimulation of its intracellular Na+-activated site, and the NKCC1-mediated uptake by inhibition of depolarization- and L-channel-mediated Ca2+ uptake. Drugs inhibiting the signaling pathways involved in either of these processes also abolished K+ uptake. Assuming similar in vivo characteristics, partly supported by literature data, K+-stimulated astrocytic K+ uptake must discontinue after normalization of extracellular K+. This will allow Kir1.4-mediated release and reuptake by the less powerful neuronal Na+,K+-ATPase.








Similar content being viewed by others
References
Macaulay N, Zeuthen T (2012) Glial K+ clearance and cell swelling: key roles for cotransporters and pumps. Neurochem Res 37:2299–2309
Ransom CB, Ransom BR, Sontheimer H (2000) Activity-dependent extracellular K+ accumulation in rat optic nerve: the role of glial and axonal Na+ pumps. J Physiol 522(Pt 3):427–442
Somjen GG, Kager H, Wadman WJ (2008) Computer simulations of neuron-glia interactions mediated by ion flux. J Comput Neurosci 25:349–365
Walz W (2000) Role of astrocytes in the clearance of excess extracellular potassium. Neurochem Int 36:291–300
Wang F, Smith NA, Xu Q et al (2012) Astrocytes modulate neural network activity by Ca2+-dependent uptake of extracellular K+. Sci Signal 5:ra26
Wang F, Xu Q, Wang W et al (2012) Bergmann glia modulate cerebellar Purkinje cell bistability via Ca2+-dependent K+ uptake. Proc Natl Acad Sci USA 109:7911–7916
Grisar T, Franck G, Schoffeniels E (1980) Glial control of neuronal excitability in mammals: II. Enzymatic evidence: two molecular forms of the Na+, K+-ATPase in brain. Neurochem Int 2C:311–320
Hajek I, Subbarao KV, Hertz L (1996) Acute and chronic effects of potassium and noradrenaline on Na+, K+-ATPase activity in cultured mouse neurons and astrocytes. Neurochem Int 28:335–342
Henn FA, Haljamae H, Hamberger A (1972) Glial cell function: active control of extracellular K+ concentration. Brain Res 43:437–443
Walz W (1992) Role of Na/K/Cl cotransport in astrocytes. Can J Physiol Pharmacol 70(Suppl):S260–S262
Walz W, Hertz L (1984) Intense furosemide-sensitive potassium accumulation in astrocytes in the presence of pathologically high extracellular potassium levels. J Cereb Blood Flow Metab 4:301–304
Dufour S, Dufour P, Chever O et al (2011) In vivo simultaneous intra- and extracellular potassium recordings using a micro-optrode. J Neurosci Methods 194:206–217
Bay V, Butt AM (2012) Relationship between glial potassium regulation and axon excitability: a role for glial Kir4.1 channels. Glia 60:651–660
Dinuzzo M, Mangia S, Maraviglia B et al (2012) The role of astrocytic glycogen in supporting the energetics of neuronal activity. Neurochem Res 37:2423–2438
Hof PR, Pascale E, Magistretti PJ (1988) K+ at concentrations reached in the extracellular space during neuronal activity promotes a Ca2+-dependent glycogen hydrolysis in mouse cerebral cortex. J Neurosci 8:1922–1928
Bernard C (1878) Leçons sur les phénomènes de la vie communs aux animaux et aux végétaux, Tome 1. Edité par Albert Dastre. J.-B. Baillière et fils, Paris
Walz W, Hertz L (1982) Ouabain-sensitive and ouabain-resistant net uptake of potassium into astrocytes and neurons in primary cultures. J Neurochem 39:70–77
Sykova E (1992) K+ homeostasis in the ECS. In: Sykova E (ed) Ionic and volume changes in the microenvironment of nerve and receptor cells in progress in sensory physiology. Springer, Berlin, pp 7–26
Epstein FH, Silva P (1985) Na-K-Cl cotransport in chloride-transporting epithelia. Ann N Y Acad Sci 456:187–197
Dawson DC (1987) Cellular mechanisms for K transport across epithelial cell layers. Semin Nephrol 7:185–192
Kimelberg HK, Frangakis MV (1985) Furosemide- and bumetanide-sensitive ion transport and volume control in primary astrocyte cultures from rat brain. Brain Res 361:125–134
Walz W, Hinks EC (1986) A transmembrane sodium cycle in astrocytes. Brain Res 368:226–232
Tas PW, Massa PT, Kress HG et al (1987) Characterization of an Na+/K+/Cl− cotransport in primary cultures of rat astrocytes. Biochim Biophys Acta 903:411–416
Kanaka C, Ohno K, Okabe A et al (2001) The differential expression patterns of messenger RNAs encoding K-Cl cotransporters (KCC1, 2) and Na-K-2Cl cotransporter (NKCC1) in the rat nervous system. Neuroscience 104:933–946
Mikawa S, Wang C, Shu F et al (2002) Developmental changes in KCC1, KCC2 and NKCC1 mRNAs in the rat cerebellum. Brain Res Dev Brain Res 136:93–100
Deisz RA, Lehmann TN, Horn P et al (2011) Components of neuronal chloride transport in rat and human neocortex. J Physiol 589:1317–1347
Zhang LL, Delpire E, Vardi N (2007) NKCC1 does not accumulate chloride in developing retinal neurons. J Neurophysiol 98:266–277
Pedersen SF, O’Donnell ME, Anderson SE et al (2006) Physiology and pathophysiology of Na+/H+ exchange and Na+-K+-2Cl− cotransport in the heart, brain, and blood. Am J Physiol Regul Integr Comp Physiol 291:R1–R25
Subbarao KV, Stolzenburg JU, Hertz L (1995) Pharmacological characteristics of potassium-induced, glycogenolysis in astrocytes. Neurosci Lett 196:45–48
Cai L, Du T, Song D et al (2011) Astrocyte ERK phosphorylation precedes K+-induced swelling but follows hypotonicity-induced swelling. Neuropathology 31:250–264
Hertz L, Bender AS, Woodbury DM et al (1989) Potassium-stimulated calcium uptake in astrocytes and its potent inhibition by nimodipine. J Neurosci Res 22:209–215
Peng L, Du T, Xu J et al (2012) Adrenergic and V1-ergic agonists/antagonists affecting recovery from brain trauma in the lund project act on astrocytes. Curr Signal Transduct Ther 7:43–55
MacVicar BA (1984) Voltage-dependent calcium channels in glial cells. Science 226:1345–1347
Duffy S, MacVicar BA (1994) Potassium-dependent calcium influx in acutely isolated hippocampal astrocytes. Neuroscience 61:51–61
Brown AM, Westenbroek RE, Catterall WA et al (2001) Axonal L-type Ca2+ channels and anoxic injury in rat CNS white matter. J Neurophysiol 85:900–911
Cahoy JD, Emery B, Kaushal A et al (2008) A transcriptome database for astrocytes, neurons, and oligodendrocytes: a new resource for understanding brain development and function. J Neurosci 28:264–278
Barres BA, Koroshet WJ, Chun LL et al (1990) Ion channel expression by whiteatter glia: the type-1 astrocytes. Neuron 5:527–544
Lovatt D, Sonnewald U, Waagepetersen HS et al (2007) The transcriptome and metabolic gene signature of protoplasmic astrocytes in the adult murine cortex. J Neurosci 27:12255–12266
Westenbroek RE, Bausch SB, Lin RC et al (1998) Upregulation of L-type Ca2+ channels in reactive astrocytes after brain injury, hypomyelination, and ischemia. J Neurosci 18:2321–2334
Peng L (2004) Transactivation in astrocytes as a novel mechanism of neuroprotection. In: Hertz L (ed) Non-neuronal cells of the nervous system: function and dysfunction. Elsevier, Amsterdam, pp 503–518
Mollenhauer HH, Morre DJ, Minnifield N (1992) Swelling response of golgi apparatus cisternae in cells treated with monensin is reduced by cell injury. Cell Biol Int Rep 16:217–220
Gautron S, Dos Santos G, Pinto-Henrique D et al (1992) The glial voltage-gated sodium channel: cell- and tissue-specific mRNA expression. Proc Natl Acad Sci USA 89:7272–7276
Black JA, Dib-Hajj S, Cohen S et al (1998) Glial cells have heart: rH1 Na+ channel mRNA and protein in spinal cord astrocytes. Glia 23:200–208
Watanabe E, Fujikawa A, Matsunaga H et al (2000) Nav2/NaG channel is involved in control of salt-intake behavior in the CNS. J Neurosci 20:7743–7751
Ogata N, Ohishi Y (2002) Molecular diversity of structure and function of the voltage-gated Na+ channels. Jpn J Pharmacol 88:365–377
Shimizu H, Watanabe E, Hiyama TY et al (2007) Glial Nax channels control lactate signaling to neurons for brain Na+ sensing. Neuron 54:59–72
Mobasheri A, Barrett-Jolley R, Shakibaei M et al (2005) Enigmatic roles of the epithelial sodium channel (ENaC) in articular chondrocytes and osteoblasts: mechanotransduction, sodium transport or extracellular sodium sensing? In: Kamkin A, Kiseleva I (eds) Mechanosensitivity in cells and tissues. Academia, Moscow, pp 452–464
Reinhard L, Tidow H, Clausen MJ et al (2012) Na+, K+-ATPase as a docking station: protein–protein complexes of the Na+, K+-ATPase. Cell Mol Life Sci. doi:10.1007/s00018-012-1039-9
Xue Z, Li B, Gu L et al (2010) Increased Na, K-ATPase alpha2 isoform gene expression by ammonia in astrocytes and in brain in vivo. Neurochem Int 57:395–403
Zhang L, Zhang Z, Guo H et al (2008) Na+/K+-ATPase-mediated signal transduction and Na+/K+-ATPase regulation. Fundam Clin Pharmacol 22:615–621
Juurlink BHJ, Hertz L (1992) Astrocytes. In: Boulton AA, Baker GB, Walz W (eds) Neuromethods in cell cultures. Humana Clifton, New York, pp 269–321; Internet availability in Springer Protocols, Neuroscience
Hertz L, Peng L, Lai JC (1998) Functional studies in cultured astrocytes. Methods 16:293–310
Li B, Du T, Li H et al (2008) Signalling pathways for transactivation by dexmedetomidine of epidermal growth factor receptors in astrocytes and its paracrine effect on neurons. Br J Pharmacol 154:191–203
Hertz L, Juurlink BHJ, Szuchet S (1985) Cell cultures. In: Lajtha A (ed) Handbook of neurochemistry. Plenum, New York, pp 603–661
Meier E, Hertz L, Schousboe A (1991) Neurotransmitters as developmental signals. Neurochem Int 19:1–15
Lange SC, Bak LK, Waagepetersen HS et al (2012) Primary cultures of astrocytes: their value in understanding astrocytes in health and disease. Neurochem Res 37:2569–2588
Li B, Dong L, Wang B et al (2012) Cell type-specific gene expression and editing responses to chronic fluoxetine treatment in the in vivo mouse brain and their relevance for stress-induced anhedonia. Neurochem Res 37:2380–2495
Li B, Hertz L, Peng L (2012b) Aralar mRNA and protein levels in neurons and astrocytes freshly isolated from young and adult mouse brain and in maturing cultured astrocytes. Neurochem Int 61:1325–1332
Song D, Li B, Yan EZ et al (2012) Chronic treatment with anti-bipolar drugs causes intracellular alkalinization in astrocytes, altering their functions. Neurochem Res 37:2524–2540
Deshmukh HS, Case LM, Wesselkamper SC et al (2005) Metalloproteinases mediate mucin 5AC expression by epidermal growth factor receptor activation. Am J Respir Crit Care Med 171:305–314
Laskay G, Varhelyi T, Dale RE et al (1995) Role of interleukin-3 in the regulation of intracellular K+ homeostasis in cultured murine hematopoietic cells. Biochem Biophys Res Commun 214:348–353
Andersson B, Janson V, Behnam-Motlagh P et al (2006) Induction of apoptosis by intracellular potassium ion depletion: using the fluorescent dye PBFI in a 96-well plate method in cultured lung cancer cells. Toxicol In Vitro 20:986–994
Yates SL, Fluhler EN, Lippiello PM (1992) Advances in the use of the fluorescent probe fura-2 for the estimation of intrasynaptosomal calcium. J Neurosci Res 32:255–260
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Sickmann HM, Schousboe A, Fosgerau K et al (2005) Compartmentation of lactate originating from glycogen and glucose in cultured astrocytes. Neurochem Res 30:1295–1304
Gibbs M, Lloyd H, Santa T et al (2007) Glycogen is a preferred glutamate precursor during learning in 1-day-old chick: biochemical and behavioral evidence. J Neurosci Res 85:3326–3333
Obel LF, Muller MS, Walls AB et al (2012) Brain glycogen-new perspectives on its metabolic function and regulation at the subcellular level. Front Neuroenergetics 4:3
Ma D, Wang J, Zhao Y et al (2012) Inhibition of glycogen phosphorylation induces changes in cellular proteome and signaling pathways in MIA pancreatic cancer cells. Pancreas 41:397–408
Sickmann HM, Waagepetersen HS, Schousboe A et al (2012) Brain glycogen and its role in supporting glutamate and GABA homeostasis in a type 2 diabetes rat model. Neurochem Int 60:267–275
Obel LF, Andersen KM, Bar LK et al (2012) Effects of adrenergic agents on intracellular Ca2+ homeostasis and metabolism of glucose in astrocytes with an emphasis on pyruvate carboxylation, oxidative decarboxylation and recycling: implications for glutamate neurotransmission and excitotoxicity. Neurotox Res 21:405–417
Kaufman E, Driscoll B (1992) Carbon dioxide fixation in neuronal and astroglial cells in culture. J Neurochem 58:258–262
Öz G, Tesfaye N, Kumar A et al (2012) Brain glycogen content and metabolism in subjects with type 1 diabetes and hypoglycemia unawareness. J Cereb Blood Flow Metab 32:256–263
Hertz L (2011) Astrocytic energy metabolism and glutamate formation—relevance for 13C-NMR spectroscopy and importance of cytosolic/mitochondrial trafficking. Magn Reson Imaging 29:1319–1329
Zhao Z, Hertz L, Code WE (1996) Effects of benzodiazepines on potassium-induced increase in free cytosolic calcium concentration in astrocytes: interactions with nifedipine and the peripheral-type benzodiazepine antagonist PK 11195. Can J Physiol Pharmacol 74:273–277
Ozawa E (2011) Regulation of phosphorylase kinase by low concentrations of Ca ions upon muscle contraction: the connection between metabolism and muscle contraction and the connection between muscle physiology and Ca-dependent signal transduction. Proc Jpn Acad Ser B Phys Biol Sci 87:486–508
Scemes E, Spray DC (2012) Extracellular K+ and astrocyte signaling via connexin and pannexin channels. Neurochem Res 37:2310–2316
Magistretti PJ, Morrison JH, Shoemaker WJ et al (1983) Effect of 6-hydroxydopamine lesions on norepinephrine-induced [3H]glycogen hydrolysis in mouse cortical slices. Brain Res 261:159–166
Cambray-Deakin M, Pearce B, Morrow C et al (1988) Effects of neurotransmitters on astrocyte glycogen stores in vitro. J Neurochem 51:1852–1857
Subbarao KV, Hertz L (1990) Noradrenaline induced stimulation of oxidative metabolism in astrocytes but not in neurons in primary cultures. Brain Res 527:346–349
Gibbs ME, Anderson DG, Hertz L (2006) Inhibition of glycogenolysis in astrocytes interrupts memory consolidation in young chickens. Glia 54:214–222
Hertz L, Gibbs ME (2009) What learning in day-old chickens can teach a neurochemist: focus on astrocyte metabolism. J Neurochem 109(Suppl 1):10–16
Suzuki A, Stern SA, Bozdaqi O et al (2011) Astrocyte-neuron lactate transport is required for long-term memory formation. Cell 144:810–823
Gibbs ME, Hutchinson DS (2012) Rapid turnover of glycogen in memory formation. Neurochem Res 37:2456–2463
Ito M, Spicer SS, Schulte BA (1994) Histochemical detection of glycogen and glycoconjugates in the inner ear with modified concanavalin A-horseradish peroxidase procedures. Histochem J 26:437–446
Tseng YC, Huang CJ, Chang JC et al (2007) Glycogen phosophorylase in glycogen-rich cells is involved in the energy supply for ion regulation in fish gill epithelia. Am J Physiol Requl Inteqr Comp Physiol 293:R482–R491
Tipsmark CK, Madsen SS, Borski RJ (2004) Effect of salinity on expression of branchial ion transporters in striped bass (Morone saxatilis). J Exp Zool A Comp Exp Biol 301:979–991
Chu HQ, Huang XW, Xiong H et al (2006) Role of ion channel Na-K-2Cl and alpha2 Na, K-ATPase in cochlear potassium cycling and auditory function. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi 41:692–697
Kajimura S, Hirano T, Moriyama S et al (2004) Changes in plasma concentrations of immunoreactive ouabain in the tilapia in response to changing salinity: is ouabain a hormone in fish? Gen Comp Endocrinol 135:90–99
Larre I, Lazaro A, Contreras RG, Balda MS et al (2010) Ouabain modulates epithelial cell tight junction. Proc Natl Acad Sci USA 107:11387–11392
Forshammar J, Block L, Lundborg C et al (2011) Naloxone and ouabain in ultralow concentrations restore Na+/K+-ATPase and cytoskeleton in lipopolysaccharide-treated astrocytes. J Biol Chem 286:31586–31597
Swanson RA (1992) Physiologic coupling of glial glycogen metabolism to neuronal activity in brain. Can J Physiol Pharmacol 70(Suppl):S138–S144
Carmignoto G, Pasti L, Pozzan T (1998) On the role of voltage-dependent calcium channels in calcium signaling of astrocytes in situ. J Neurosci 18:4637–4645
Choi HB, Gordon GR, Zhou N et al (2012) Metabolic communication between astrocytes and neurons via bicarbonate-responsive soluble adenylyl cyclase. Neuron 75:1094–1104
Acknowledgments
The authors of DiNuzzo et al. [14] are cordially thanked for their contemplative suggestion that glycogenolysis may be involved in astrocytic K+ uptake, without which this study would not have been undertaken. Our study was supported by Grant No. 31171036 to L. P. from the National Natural Science Foundation of China.
Conflict of interest
Nothing to report.
Author information
Authors and Affiliations
Corresponding author
Additional information
Junnan Xu and Dan Song contributed equally to this work.
Rights and permissions
About this article
Cite this article
Xu, J., Song, D., Xue, Z. et al. Requirement of Glycogenolysis for Uptake of Increased Extracellular K+ in Astrocytes: Potential Implications for K+ Homeostasis and Glycogen Usage in Brain. Neurochem Res 38, 472–485 (2013). https://doi.org/10.1007/s11064-012-0938-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-012-0938-3