Abstract
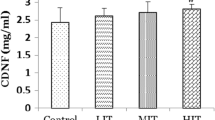
There are conflicts between the effects of free radical over-production induced by exercise on neurotrophins and brain oxidative metabolism. The objective of this study was to investigate the effects of intense physical training on brain-derived neurotrophic factor (BDNF) levels, COX activity, and lipoperoxidation levels in mice brain cortex. Twenty-seven adult male CF1 mice were assigned to three groups: control untrained, intermittent treadmill exercise (3 × 15 min/day) and continuous treadmill exercise (45 min/day). Training significantly (P < 0.05) increased citrate synthase activity when compared to untrained control. Blood lactate levels classified the exercise as high intensity. The intermittent training significantly (P < 0.05) reduced in 6.5% the brain cortex COX activity when compared to the control group. BDNF levels significantly (P < 0.05) decreased in both exercise groups. Besides, continuous and intermittent exercise groups significantly (P < 0.05) increased thiobarbituric acid reactive species levels in the brain cortex. In summary, intense exercise promoted brain mitochondrial dysfunction due to decreased BDNF levels in the frontal cortex of mice.



Similar content being viewed by others
Abbreviations
- BDNF:
-
Brain-derived neurotrophic factor
- COX:
-
Cytochrome c oxidase
- CREB:
-
Cyclic AMP response element-binding protein
- CS:
-
Citrate synthase
- ETC:
-
Electron transport chain
- mRNA:
-
Messenger RNA
- mtDNA:
-
Mitochondrial DNA
- NMDA:
-
N-methyl-d-aspartate receptor
- TBARS:
-
Thiobarbituric acid reactive species
- ROS:
-
Reactive oxygen species
References
Aguiló A, Tauler P, Fuentespina E, et al (2005) Antioxidant response to oxidative stress induced by intense exercise. Physiol Behav 84:1–7
Alp PR, Newsholme EA, Zammit VA (1976) Activities of Citrate Synthase and NAD+-Linked and NADP+-Linked Isocitrate Dehydrogenase in Muscle from Vertebrates and Invertebrates. Biochem J 154:689–700
Albeck DS, Beck KD, Kung LH et al (2005) Leverpress escape/avoidance training increases neurotrophin levels in rat brain. Integr Physiol Behav Sci 1:28–34
Arida RM, Scorza CA, Silva AV et al (2004) Differential effects of spontaneous versus forced exercise in rats on the staining of parvalbumin-positive neurons in the hippocampal formation. Neurosci Lett 364:135–138
Behl C (2005) Oxidative stress in Alzheimer’s disease: implications for prevention and therapy. Subcell Biochem 38:65–78
Billat VL, Mouisel E, Roblot N et al (2005) Inter- and intrastrain variation in mouse critical running speed. J Appl Physiol 4:1258–1263
Briones TL, Suh E, Jozsa L et al (2005) Changes in number of synapses and mitochondria in presynaptic terminals in the dentate gyrus following cerebral ischemia and rehabilitation training. Brain Res 1:51–57
Calabrese V, Lodi TR, Tonon C et al (2005) Oxidative stress, mitochondrial dysfunction and cellular stress response in Friedreich’s ataxia. J Neurol Sci 233:145–162
Colcombe SJ, Kramer AF, Erickson KI et al (2004) Cardiovascular fitness, cortical plasticity, and aging. Proc Natl Acad Sci USA 101:3316–3321
Cosķun S, Gonul B, Guzel NA et al (2005) The effects of vitamin C supplementation on oxidative stress and antioxidant content in the brains of chronically exercised rats. Mol Cell Biochem 1–2:135–138
Cotman CW, Berchtold NC, Adlard PA et al (2005) Exercise and the Brain. In: Mooren FC, Völker K (eds) Molecular and cellular exercise physiology. Champaign, IL, USA, pp 331–341
Cotman CW, Berchtold NC (2002) Exercise: a behavioral intervention to enhance brain health and plasticity. Trends Neurosci 25:295–301
Cronin JB, McNair PJ, Marshall RN (2002) Is velocity-specific strength training important in improving functional performance? J Sports Med Phys Fitness 3:267–273
Denadai BS (2000) Avaliação aeróbia. In: Denadai BS (ed) Avaliação aeróbia, vol. 1. Motrix, Rio Claro-Brazil, pp 22–24
Ding Q, Vaynman S, Akhavan M et al (2006) Insulin-like growth factor I interfaces with brain-derived neurotrophic factor-mediated synaptic plasticity to modulate aspects of exercise-induced cognitive function. Neuroscience 3:823–833
Ding Y, Li J, Luan X et al (2004) Exercise pre-conditioning reduces brain damage in ischemic rats that may be associated with regional angiogenesis and cellular overexpression of neurotrophin. Neuroscience 3:583–591
Dohm MR, Hayes JP, Garland T Jr (2001) The quantitative genetics of maximal and basal rates of oxygen consumption in mice. Genetics 159:267–277
Draper HH, Hadley M (1990) Malondialdehyde determination as index of lipid peroxidation. Meth Enzymol 186:421–431
Duncan AJ, Heales SJ (2005) Nitric oxide and neurological disorders. Mol Aspects Med 1–2:67–96
Ebadi M, Leuschen MP, el Refaey H et al (1996) The antioxidant properties of zinc and metallothionein. Neurochem Int 2:159–66
Fauchex BA, Martin ME, Beaumont C et al (2003) Neuromelanin associated redox-active iron is increased in the substantia nigra of patients with Parkinson’s Disease. J Neurochem 5:1142–1148
Floyd RA (1999) Antioxidants, oxidative stress, and neurological disorders. Exp Biol Med 222:236–245
Garifoli A, Cardile V, Maci T et al (2003) Exercise increases cytochrome oxidase activity in specific cerebellar areas of the rat. Arch Ital Biol 4:181–187
Hellweg R, von Richthofen S, Anders D et al (1998) The time course of nerve growth factor content in different neuropsychiatric diseases—a unifying hypothesis. J Neural Transm 8–9:871–903
Hessel E, Haberland A, Muller M et al (2000) Oxygen radical generation of neutrophils: a reason for oxidative stress during marathon running? Clin Chim Acta 1–2:145–156
Ide K, Secher NH (2000) Cerebral blood flow and metabolism during exercise. Prog Neurobiol 4:397–414
Jacobson J, Duchen MR, Hothersall J et al (2005). Induction of mitochondrial oxidative stress in astrocytes by nitric oxide precedes disruption of energy metabolism. J Neurochem 2:388–395
Jolitha AB, Subramanyam MVV, Devi S (2006) Modification by vitamin E and exercise of oxidative stress in regions of aging rat brain: studies on superoxide dismutase isoenzymes and protein oxidation status. Exp Gerontol 41:753–763
Kann O, Kovacs R (2006) Mitochondria and Neuronal Activity. Am J Physiol Cell Physiol 2:641–657
Keeney PM, Xie J, Capaldi RA et al (2006) Parkinson’s disease brain mitochondrial complex I has oxidatively damaged subunits and is functionally impaired and misassembled. J Neurosci 19:5256–264
Kim MW, Bang MS, Han TR, Ko YJ, Yoon BW, Kim JH, Kang LM, Lee KM, Kim MH (2005) Exercise increased BDNF and trkB in the contralateral hemisphere of the ischemic rat brain. Brain Res 1:16–21
Koteja P, Swallow JG, Carter PA et al (1999) Energy cost of wheel running in house mice: implications for coadaptation of locomotion and energy budgets. Physiol Biochem Zool 2:238–249
Kress J, Dineley KE, Reynolds IJ (2002) The relationship between intracellular free iron and cell injury in cultured neurons, astrocytes, and oligodendrocytes. J Neurosci 14:5848–5855
Lambert MI, Van Zyl C, Jaunky R et al (1996) Tests of running performance do not predict subsequent spontaneous running in rats. Physiol Behav 1:171–176
Lewis MH (2004) Environmental complexity and central nervous system development and function. Ment Retard Dev Disabil Res Ver 2:91–95
Light KE, Ge Y, Belcher SM (2001) Early postnatal ethanol exposure selectively decreases BDNF and truncated TrkB-T2 receptor mRNA expression in the rat cerebellum. Brain Res Mol Brain Res 1:46–55
Lightfoot JT, Turner MJ, Debate KA et al (2001) Interstrain variation in murine aerobic capacity. Med Sci Sports Exerc 33:2053–2057
Liu J, Yeo HC, Overvik-Douki E et al (2000) Chronically and acutely exercised rats: biomarkers of oxidative stress and endogenous antioxidants. J Appl Physiol 1:21–28
Lowry OH, Rosebough NG, Farr AL et al (1951) Protein measurement with the folin phenol reagent. J Biol Chem 193:265–275
Lu C, Chan SL, Haughey N et al (2001) Selective and biphasic effect of the membrane lipid peroxidation product 4-hydroxy-2,3-nonenal on N-methyl-d-aspartate channels. J Neurochem 3:577–589
Mader A, Heck (1986) A theory of metabolic origin of the anaerobic threshold. Int J Sports Med 7:45–65
Markesbery WR (1997) Oxidative stress hypothesis in Alzheimer’s disease. Free Radic Biol Med 1:134–147
Maswood N, Young J, Tilmont E et al (2004) Caloric restriction increases neurotrophic factor levels and attenuates neurochemical and behavioral deficits in a primate model of Parkinson’s disease. PNAS 101:18171–18176
Mattson MP, Liu D (2002) Energetics and oxidative stress in synaptic plasticity and neurodegenerative disorders. Neuromolecular Med 2:215–231
Mondon CE, Dolkas CB, Sims C et al (1985) Spontaneous running activity in male rats: effect of age. J Appl Physiol 5:1553–1557
Navarro A (2004) Mitochondrial enzyme activities as biochemical markers of aging. Mol Aspects Med 25:37–48
Navarro A, Del Pino MJS, Gomez C et al (2002) Behavioral dysfunction, brain oxidative stress, and impaired mitochondrial electron transfer in aging mice. Am J Physiol Regul Integr Comp Physiol 282:985–992
Nied RJ, Franklin B (2002) Promoting and prescribing exercise for the elderly. Am Fam Physician 3:419–426
Ogonovszky H, Berkes I, Kumagai S et al (2005) The effects of moderate-, strenuous- and over-training on oxidative stress markers, DNA repair, and memory, in rat brain. Neurochem Int 46:635–640
Özkaya YG, Agar A, Yargicoglu P et al (2002) The effect of exercise on brain antioxidant status of diabetic rats. Diabetes Metab 5:377–384
Pinho RA, Andrades ME, Oliveira MR et al (2006) Imbalance in SOD/CAT activities in rats skeletal muscles submitted to treadmill training exercise. Cell Biol Int 10:848–853
Radák Z, Toldy A, Szabo Z et al (2006) The effects of training and detraining on memory, neurotrophins and oxidative stress markers in rat brain. Neurochem Int 4:387–392
Redila VA, Christie BR (2006) Exercise-induced changes in dendritic structure and complexity in the adult hippocampal dentate gyrus. Neuroscience 4:1299–1307
Rizzardini M, Lupi M, Mangolini A et al (2006) Neurodegeneration induced by complex I inhibition in a cellular model of familial amyotrophic lateral sclerosis. Brain Res Bull 4:465–474
Roceri M, Hendriks W, Racagni G et al (2002) Early maternal deprivation reduces the expression of BDNF and NMDA receptor subunits in rat hippocampus. Mol Psychiatry 6:609–616
Rustin P, Chretien D, Bourgeron T et al (1994) Biochemical and molecular investigations in respiratory chain deficiencies. Clin Chim Acta 228:35–51
Sastre J, Asensi M, Gasco E et al (1992) Exhaustive physical exercise causes oxidation of glutathione status in blood: prevention by antioxidant administration. Am J Physiol 5 Pt 2:992–995
Sen CK, Packer L (2000) Thiol homeostasis and supplements in physical exercise. Am J Nutr 72:653–669
Shibuya K, Tanaka J, Kuboyama N et al (2004) Cerebral oxygenation during intermittent supramaximal exercise. Respir Physiol Neurobiol 2:165–172
Simonian NA, Coyle JT (1996) Oxidative stress in neurodegenerative diseases. Annu Rev Pharmacol Toxicol 36:83–106
Siu PM, Donley DA, Bryner RW et al (2003) Citrate Synthase expression and enzyme activity after endurance training in cardiac and skeletal muscles. J Appl Physiol 94:555–560
Somani SM, Ravi R, Rybak LP (1995) Effect of exercise training on antioxidant system in brain regions of rat. Pharmacol Biochem Behav 4:635–639
Sureda A, Tauler P, Aguiló A et al (2005) Relation between oxidative stress markers and antioxidant endogenous defences during exhaustive exercise. Free Radic Res 12:1317–1324
Suzuki K, Machida K, Kariya M (1992) Conditions for low-intensity voluntary wheel running in rats and its chronic effects on health indexes. Nippon Eiseigaku Zasshi 5:939–951
Suzuki M, Katamine S, Tatsumi S (1983) Exercise-induced enhancement of lipid peroxide metabolism in tissues and their transference into the brain in rat. J Nutr Sci Vitaminol 2:141–151
Swain RA, Harris AB, Wiener EC et al (2003) Prolonged exercise induces angiogenesis and increases cerebral blood volume in primary motor cortex of the rat. Neuroscience 4:1037–1046
Tauler P, Aguiló A, Gimeno I et al (2006) Response of blood cell antioxidant enzyme defences to antioxidant diet supplementation and to intense exercise. Eur J Nutr 4:187–195
Tsai K, Hsu TG, Hsu KM et al (2001) Oxidative DNA damage in human peripheral leukocytes induced by massive aerobic exercise. Free Radic Biol Med 11:1465–1472
Vaynman S, Ying Z, Wu A et al (2006a) Coupling energy metabolism with a mechanism to support brain-derived neurotrophic factor-mediated synaptic plasticity. Neuroscience 4:1221–1234
Vaynman S, Yinga Z, Yina D et al (2006b) Exercise differentially regulates synaptic proteins associated to the function of BDNF. Brain Res 1070:124–130
Voltarelli FA, Gobatto CA, Mello MAR (2002) Determination of anaerobic threshold in rats using the lactate minimum test. Braz J Med Biol Res 35:1389–1394
Zhu SW, Pham TM, Aberg E et al (2006) Neurotrophin levels and behaviour in BALB/c mice: impact of intermittent exposure to individual housing and wheel running. Behav Brain Res 1:1–8
Wu A, Ying Z, Gomez-Pinilla F (2004) The interplay between oxidative stress and brain-derived neurotrophic factor modulates the outcome of a saturated fat diet on synaptic plasticity and cognition. Eur J Neurosci 19:1699–1707
Acknowledgments
This research was supported by grants from CNPq/MCT (Brazil), CAPES/MEC (Brazil), UNESC (Brazil) and FAPESC (Brazil).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Aguiar, A.S., Tuon, T., Pinho, C.A. et al. Intense Exercise Induces Mitochondrial Dysfunction in Mice Brain. Neurochem Res 33, 51–58 (2008). https://doi.org/10.1007/s11064-007-9406-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-007-9406-x