Abstract
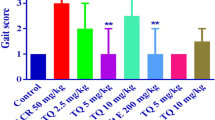
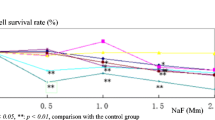
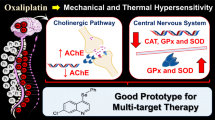
Occupational exposure and experimental intoxication with n-hexane or its metabolite 2,5-hexanedione (HD) produce a central-peripheral neuropathy. However, the mechanism remains unknown. We hypothesized that HD affected the expression of Bcl-2, Bax and Caspase-3 in the central nervous system (CNS) and the peripheral nervous system (PNS). Male adult Wistar rats were administered by intraperitoneal injection at a dosage of 200 or 400 mg/kg HD, five days per week for 8 weeks. Samples of the cerebral cortex, cerebellum, spinal cord and sciatic nerves were collected and examined for Bcl-2, Bax and Caspase-3 expression using Western blotting. Subchronic exposure to HD resulted in significantly increased expression of both anti-apoptotic protein Bcl-2 and pro-apoptotic protein Bax and Caspase-3 in cerebral cortex and cerebellum, which exhibited a dose-dependent pattern. Though little change was detected in spinal cord, our results showed that the expression of Bcl-2, Bax and Caspase-3 was markedly enhanced in the sciatic nerves. These findings suggested that the changes of apoptosis-related protein level in rat nerve tissues were associated with the intoxication of HD, which might be involved in early molecular regulatory mechanism of apoptosis in the HD-induced neuropathy.




Similar content being viewed by others
References
Spencer PS, Schaumburg HH (1977) Neurotoxic properties of certain aliphatic hexacarbons. Proc R Soc Med 70:37–38
Couri D, Milks M (1982) Toxicity and metabolism of the neurotoxic hexacarbons n-hexane, 2-hexanone, and 2,5-hexanedione. Ann Rev Pharmacol Toxicol 22:145–166
Graham DG, Amarnath V, Valentine WM et al (1995) Pathogenetic studies of hexane and carbon disulfide neurotoxicity. Crit Rev Toxicol 25:91–112
Lehning EJ, Jortner BS, Fox JH et al (2000) gamma-diketone peripheral neuropathy. I. Quality morphometric analyses of axonal atrophy and swelling. Toxicol Appl Pharmacol 165(2):127–140
Sterman AB, Sposito N (1985) 2,5-Hexanedione and acrylamide produce reorganization of motoneuron perikarya. Neuropathol Appl Neurobiol 11(3):201–212
Sterman AB (1982) Cell body remodeling during dying-back axonopathy: DRG changes during advanced disease. J Neuropathol Exp Neurol 41(4):400–411
Moretto G, Monaco S, Passarin MG et al (1991) Cytoskeletal changes induced by 2,5-hexanedione on developing human neurons in vitro. Arch Toxicol 65(5):409–413
Carney R, Dardis C, Cullen WK et al (2002) Early spatial memory deficit induced by 2,5-hexanedione in the rat. Toxicol Lett 128(1–3):107–115
De Rojas TC, Goldstein BD (1990) Lack of evidence for the size principle of selective vulnerability of axons in toxic neuropathies. I. The effects of subcutaneous injections of 2,5-hexanedione on behavior and muscle spindle function. Toxicol Appl Pharmacol 104(1):47–58
Mateus ML, dos Santos AP, Batoreu MC (2002) Evidence of zinc protection against 2,5-hexanedione neurotoxicity: correlation of neurobehavioral testing with biomarkers of excretion. Neurotoxicology 23(6):747–754
Ogawa Y, Shimizu H, Kim SU (1996) 2,5-Hexanedione induced apoptosis in cultured mouse DRG neurons. Int Arch Occup Environ Health 68:495–497
Strange P, Moller A, Ladefoged O et al (1991) Total number and mean cell volume of neocortical neurons in rats exposed to 2,5-hexanedione with and without acetone. Neurotoxicol Teratol 13(4):401–406
Backstrom B, Dumanski JP, Collins VP (1990) The effects of 2,5-hexanedione on the retina of albino rats. Neurotoxicology 11(1):47–55
Cavanagh JB (1964) The significance of the “dying back” process in experimental and human neurological disease. Int Rev Exp Pathol 3:219–267
Lo AC, Houenou LJ, Oppenheim RW (1995) Apoptosis in the nervous system: morphological features, methods, pathology, and prevention. Arch Histol Cytol 58(2):139–149
Sima AA, Bouchier M, Christensen H (1983) Axonal atrophy in sensory nerves of the diabetic BB-Wistar rat: a possible early correlate of human diabetic neuropathy. Ann Neurol 13(3):264–272
Canesi M, Perbellini L, Maestri L et al (2003) Poor metabolization of n-hexane in Parkinson’s disease. J Neurol 250(5):556–560
Jenner P (1998) Oxidative mechanisms in nigral cell death in Parkinson’s disease. Mov Disord 13(Suppl 1):24–34
Jellinger KA (2001) Cell death mechanisms in neurodegeneration. J Cell Mol Med 5(1):1–17
Lopez E, Pozas E, Rivera R et al (1999) Bcl-2, Bax and Bcl-x expression following kainic acid administration at convulsant doses in the rat. Neuroscience 91(4):1461–1470
Laemmli UK et al (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227(5259):680–685
Zha H, Aime-Sempe C, Sato T et al (1996) Proapoptotic protein Bax heterodimerizes with Bcl-2 and homodimerizes with Bax via a novel domain (BH3) distinct from BH1 and BH2. J Biol Chem 271(13):7440–7444
Garcia I, Martinou I, Tsujimoto Y et al (1992) Prevention of programmed cell death of sympathetic neurons by the bcl-2 proto-oncogene. Science 258(5080):302–304
Kane DJ, Sarafian TA, Anton R et al (1993) Bcl-2 inhibition of neural death: decreased generation of reactive oxygen species. Science 262(5137):1274–1277
Chen RW, Chuang DM (1999) Long term lithium treatment suppresses p53 and Bax expression but increases Bcl-2 expression. A prominent role in neuroprotection against excitotoxicity. J Biol Chem 274:6039–6042
Wei H, Leeds P, Chen RW et al (2000) Neuronal apoptosis induced by pharmacological concentrations of 3-hydroxykynurenine: characterization and protection by dantrolene and Bcl-2 overexpression. J Neurochem 75(1):81–90
Marshall KA, Daniel SE, Cairns N et al (1997) Upregulation of the anti-apoptotic protein Bcl-2 may be an early event in neurodegeneration: studies on Parkinson’s and incidental Lewy body disease. Biochem Biophys Res Commun 240(1):84–87
Mogi M, Harada M, Kondo T et al (1996) Bcl-2 protein is increased in the brain from Parkinsonian patients. Neurosci Lett 215(2):137–139
Satou T, Cummings BJ, Cotman CW (1995) Immunoreactivity for Bcl-2 protein within neurons in the Alzheimer’s disease brain increases with disease severity. Brain Res 697(1–2):35–43
Kitamura Y, Shimohama S, Kamoshima W et al (1998) Alteration of proteins regulating apoptosis, Bcl-2, Bcl-x, Bax, Bak, Bad, ICH-1 and CPP32, in Alzheimer’s disease. Brain Res 780(2):260–269
Fan H, Favero M, Vogel MW (2001) Elimination of Bax expression in mice increases cerebellar purkinje cell numbers but not the number of granule cells. J Comp Neurol 436(1):82–91
Deckwerth TL, Elliott JL, Knudson CM et al (1996) BAX is required for neuronal death after trophic factor deprivation and during development. Neuron 17(3):401–411
White FA, Keller-Peck CR, Knudson CM et al (1998) Widespread elimination of naturally occurring neuronal death in Bax-deficient mice. J Neurosci 18(4):1428–1439
Kaufmann SH, Desnoyers S, Ottaviano Y et al (1993) Specific proteolytic cleavage of poly (ADP-ribose) polymerase: an early marker of chemotherapy-induced apoptosis. Cancer Res 53(17):3976–3985
Sastry PS, Rao KS (2000) Apoptosis and the nervous system. J Neurochem 74(1):1–20
Jellinger KA, Stadelmann CH (2000) The enigma of cell death in neurodegenerative disorders. J Neural Transm Suppl 60:21–36
MacGibbon GA, Lawlor PA, Walton M et al (1997) Expression of Fos, Jun, and Krox family proteins in Alzheimer’s disease. Exp Neurol 147(2):316–332
Stadelmann C, Bruck W, Bancher C et al (1998) Alzheimer disease: DNA fragmentation indicates increased neuronal vulnerability, but not apoptosis. J Neuropathol Exp Neurol 57(5):456–464
Torp R, Su JH, Deng G et al (1998) GADD45 is induced in Alzheimer’s disease, and protects against apoptosis in vitro. Neurobiol Dis 5(4):245–252
Anderson AJ, Stoltzner S, Lai F et al (2000) Morphological and biochemical assessment of DNA damage and apoptosis in Down syndrome and Alzheimer disease, and effect of postmortem tissue archival on TUNEL. Neurobiol Aging 21(4):511–524
Hartmann A, Hunot S, Michel PP et al (2000) Caspase-3: a vulnerability factor and final effector in apoptotic death of dopaminergic neurons in Parkinson’s disease. Proc Natl Acad Sci USA 97(6):2875–2880
Jellinger KA (2000) Cell death mechanisms in Parkinson’s disease. J Neural Transm 107(1):1–29
Vukosavic S, Dubois-Dauphin M, Romero N et al (1999) Bax and Bcl-2 interaction in a transgenic mouse model of familial amyotrophic lateral sclerosis. J Neurochem 73(6):2460–2468
Merry DE, Korsmeyer SJ (1997) Bcl-2 gene family in the nervous system. Annu Rev Neurosci 20:245–267
Keane RW, Srinivasan A, Foster LM et al (1997) Activation of CPP32 during apoptosis of neurons and astrocytes. J Neurosci Res 48(2):168–180
Chen J, Nagayama T, Jin K et al (1998) Induction of caspase-3-like protease may mediate delayed neuronal death in the hippocampus after transient cerebral ischemia. J Neurosci 18(13):4914–4928
Ni B, Wu X, Su Y et al (1998) Transient global forebrain ischemia induces a prolonged expression of the caspase-3 mRNA in rat hippocampal CA1 pyramidal neurons. J Cereb Blood Flow Metab 18(3):248–256
Ladefoged O, Roswall K, Larsen JJ et al (1994) Acetone potentiation and influence on the reversibility of 2,5-hexanedione-induced neurotoxicity studied with behavioural and morphometric methods in rats. Pharmacol Toxicol 74(4–5):294–299
Sager PR (1989) Cytoskeletal effects of acrylamide and 2,5-hexanedione: selective aggregation of vimentin filaments. Toxicol Appl Pharmacol 97(1):141–155
Powell HC, Koch T, Garrett R et al (1978) Schwann cell abnormalities in 2,5-hexanedione neuropathy. J Neurocytol 7(4):517–528
Acknowledgements
This work was supported by grants from the Ministry of Science and Technology of China (No. 2002CB512907), and National Natural Science Foundation of China (No. 30271138).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cui, N., Li, S., Zhao, X. et al. Expression of Bcl-2, Bax and Caspase-3 in Nerve Tissues of Rats Chronically Exposed to 2,5-hexanedione. Neurochem Res 32, 1566–1572 (2007). https://doi.org/10.1007/s11064-007-9359-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-007-9359-0