Abstract
Background
Glioma is a type of malignant cancer that affect the central nervous system. New predictive biomarkers have been investigated in recent years, but the clinical prognosis for glioma remains poor. The function of CPLX2 in glioma and the probable molecular mechanism of tumor suppression were the focus of this investigation.
Methods
The glioma transcriptome profile was downloaded from The Cancer Genome Atlas (TCGA) and Chinese Glioma Genome Atlas (CGGA) databases for analysis of CPLX2 expression in glioma. RT-qPCR was performed to detect the expression of CPLX2 in 68 glioma subjects who have been followed up. Kaplan-Meier survival analyses were conducted to assess the effect of CPLX2 on the prognosis of glioma patients. The knockdown and overexpressed cell lines of CPLX2 were constructed to investigate the impact of CPLX2 on glioma. The cell growth, colony formation, and tumor formation in xenograft were performed.
Results
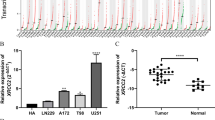
The expression of CPLX2 was downregulated in glioma and was negatively correlated with the grade of glioma. The higher expression of CPLX2 predicted a longer survival, as indicated by the analysis of Kaplan-Meier survival curves. Overexpressed CPLX2 impaired tumorigenesis in glioma progression both in vivo and in vitro. Knocking down CPLX2 promoted the proliferation of glioma cells. The analysis of GSEA and co-expression analysis revealed that CPLX2 may affect the malignancy of glioma by regulating the hypoxia and inflammation pathways.
Conclusions
Our data indicated that CPLX2 functions as a tumor suppressor and could be used as a potential prognostic marker in glioma.





Similar content being viewed by others
Data availability
The datasets used in the present work are included in the current manuscript. These data are available in TCGA (https://portal.gdc.cancer.gov/) and CGGA (http://www.cgga.org. cn) databases. The other data analyzed during this study are available from the corresponding author upon reasonable request.
Abbreviations
- CPLX1:
-
Complexion1
- CPLX2:
-
Complexion2
- WHO:
-
World Health Organization
- OS:
-
overall survival
- RT-qPCR:
-
quantitative real-time polymerase chain reaction
- WB:
-
Western blot
- TCGA:
-
The Cancer Genome Atlas
- GSEA:
-
gene set enrichment analysis
- CGGA:
-
Chinese Glioma Genome Atlas
References
Schwartzbaum JA, Fisher JL, Aldape KD, Wrensch M (2006) Epidemiology and molecular pathology of glioma. Nat Clin Pract Neurol 2(9):494–503 quiz 491 p following 516
De Vleeschouwer S, Bergers G (2017) : Glioblastoma: To Target the Tumor Cell or the Microenvironment? In: Glioblastoma Edited by De Vleeschouwer S. Brisbane (AU): Codon Publications Copyright: The Authors.;
Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, Wiestler OD, Kleihues P, Ellison DW (2016) The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol 131(6):803–820
Ostrom QT, Cioffi G, Gittleman H, Patil N, Waite K, Kruchko C, Barnholtz-Sloan JS (2019) CBTRUS Statistical Report: primary brain and other Central Nervous System tumors diagnosed in the United States in 2012–2016. Neuro Oncol 21(Suppl 5):v1–v100
Sant M, Minicozzi P, Lagorio S, Børge Johannesen T, Marcos-Gragera R, Francisci S (2012) Survival of European patients with central nervous system tumors. Int J Cancer 131(1):173–185
Tan AC, Ashley DM, López GY, Malinzak M, Friedman HS, Khasraw M (2020) Management of glioblastoma: state of the art and future directions. Cancer J Clin 70(4):299–312
Melia TJ Jr. (2007) Putting the clamps on membrane fusion: how complexin sets the stage for calcium-mediated exocytosis. FEBS Lett 581(11):2131–2139
Tang J, Maximov A, Shin OH, Dai H, Rizo J, Südhof TC (2006) A complexin/synaptotagmin 1 switch controls fast synaptic vesicle exocytosis. Cell 126(6):1175–1187
Komatsu H, Kakehashi A, Nishiyama N, Izumi N, Mizuguchi S, Yamano S, Inoue H, Hanada S, Chung K, Wei M et al (2013) Complexin-2 (CPLX2) as a potential prognostic biomarker in human lung high grade neuroendocrine tumors. Cancer Biomark 13(3):171–180
Gu W, Ren JH, Zheng X, Hu XY, Hu MJ (2019) Comprehensive analysis of expression profiles of long non–coding RNAs with associated ceRNA network involved in gastric cancer progression. Mol Med Rep 20(3):2209–2218
Wei B, Wang R, Wang L, Du C (2020) Prognostic factor identification by analysis of the gene expression and DNA methylation data in glioma. Math Biosci Engineering: MBE 17(4):3909–3924
Hass J, Walton E, Kirsten H, Turner J, Wolthusen R, Roessner V, Sponheim SR, Holt D, Gollub R, Calhoun VD et al (2015) Complexin2 modulates working memory-related neural activity in patients with schizophrenia. Eur Arch Psychiatry Clin NeuroSci 265(2):137–145
Radyushkin K, El-Kordi A, Boretius S, Castaneda S, Ronnenberg A, Reim K, Bickeböller H, Frahm J, Brose N, Ehrenreich H (2010) Complexin2 null mutation requires a ‘second hit’ for induction of phenotypic changes relevant to schizophrenia. Genes Brain Behav 9(6):592–602
Gibson HE, Reim K, Brose N, Morton AJ, Jones S (2005) A similar impairment in CA3 mossy fibre LTP in the R6/2 mouse model of Huntington’s Disease and in the complexin II knockout mouse. Eur J Neurosci 22(7):1701–1712
Chung YS, Choo BKM, Ahmed PK, Othman I, Shaikh MF (2020) : Orthosiphon Stamineus proteins Alleviate Pentylenetetrazol-Induced seizures in zebrafish. Biomedicines 8(7)
Shi Y, Tao Y, Jiang Y, Xu Y, Yan B, Chen X, Xiao L, Cao Y (2012) Nuclear epidermal growth factor receptor interacts with transcriptional intermediary factor 2 to activate cyclin D1 gene expression triggered by the oncoprotein latent membrane protein 1. Carcinogenesis 33(8):1468–1478
Jiang Y, Yan B, Lai W, Shi Y, Xiao D, Jia J, Liu S, Li H, Lu J, Li Z et al (2015) Repression of hox genes by LMP1 in nasopharyngeal carcinoma and modulation of glycolytic pathway genes by HoxC8. Oncogene 34(50):6079–6091
Mao C, Wang X, Liu Y, Wang M, Yan B, Jiang Y, Shi Y, Shen Y, Liu X, Lai W et al (2018) A G3BP1-Interacting lncRNA promotes ferroptosis and apoptosis in Cancer via Nuclear sequestration of p53. Cancer Res 78(13):3484–3496
Yan B, Liu S, Shi Y, Liu N, Chen L, Wang X, Xiao D, Liu X, Mao C, Jiang Y et al (2018) Activation of AhR with nuclear IKKα regulates cancer stem-like properties in the occurrence of radioresistance. Cell Death Dis 9(5):490
Makuuchi R, Terashima M, Kusuhara M, Nakajima T, Serizawa M, Hatakeyama K, Ohshima K, Urakami K, Yamaguchi K (2017) Comprehensive analysis of gene mutation and expression profiles in neuroendocrine carcinomas of the stomach. Biomed Res (Tokyo Japan) 38(1):19–27
Dono A, Ballester LY, Primdahl D, Esquenazi Y, Bhatia A (2021) IDH-Mutant Low-grade glioma: advances in molecular diagnosis, management, and future directions. Curr Oncol Rep 23(2):20
Chen R, Smith-Cohn M, Cohen AL, Colman H (2017) Glioma subclassifications and their clinical significance. Neurotherapeutics: The Journal of the American Society for Experimental NeuroTherapeutics 14(2):284–297
Glynn D, Gibson HE, Harte MK, Reim K, Jones S, Reynolds GP, Morton AJ (2010) Clorgyline-mediated reversal of neurological deficits in a complexin 2 knockout mouse. Hum Mol Genet 19(17):3402–3412
Tsuru E, Oryu K, Sawada K, Nishihara M, Tsuda M (2019) Complexin 2 regulates secretion of immunoglobulin in antibody-secreting cells. Immun Inflamm Dis 7(4):318–325
Pan H, Oliveira B, Saher G, Dere E, Tapken D, Mitjans M, Seidel J, Wesolowski J, Wakhloo D, Klein-Schmidt C et al (2019) Uncoupling the widespread occurrence of anti-NMDAR1 autoantibodies from neuropsychiatric Disease in a novel autoimmune model. Mol Psychiatry 24(10):1489–1501
Eissa N, Hussein H, Hendy GN, Bernstein CN, Ghia JE (2018) Chromogranin-A and its derived peptides and their pharmacological effects during intestinal inflammation. Biochem Pharmacol 152:315–326
Mahata SK, Corti A (2019) Chromogranin A and its fragments in cardiovascular, immunometabolic, and cancer regulation. Ann N Y Acad Sci 1455(1):34–58
Chen X, Zhao MZ, Miao BP, Liu ZQ, Yang G, Liu JQ, Yang PC, Song JP (2020) Inhibition of Bcl2L12 attenuates Eosinophilia-related inflammation in the heart. Front Immunol 11:1955
Varghese JF, Patel R, Yadav UCS (2019) Sterol regulatory element binding protein (SREBP) -1 mediates oxidized low-density lipoprotein (oxLDL) induced macrophage foam cell formation through NLRP3 inflammasome activation. Cell Signal 53:316–326
Uğurel E, Şehitoğlu E, Tüzün E, Kürtüncü M, Çoban A, Vural B (2016) Increased complexin-1 and decreased miR-185 expression levels in Behçet’s Disease with and without neurological involvement. Neurol Sciences: Official J Italian Neurol Soc Italian Soc Clin Neurophysiol 37(3):411–416
Frontinan-Rubio J, Llanos-Gonzalez E, Garcia-Carpintero S, Peinado JR, Ballesteros-Yanez I, Rayo MV, de la Fuente J, Perez-Garcia VM, Perez-Romasanta LA, Malumbres M et al (2023) CoQ(10) reduces glioblastoma growth and infiltration through proteome remodeling and inhibition of angiogenesis and inflammation. Cell Oncol (Dordr) 46(1):65–77
Zhu C, Kros JM, Cheng C, Mustafa D (2017) The contribution of tumor-associated macrophages in glioma neo-angiogenesis and implications for anti-angiogenic strategies. Neuro Oncol 19(11):1435–1446
Durrani IA, Bhatti A, John P (2021) The prognostic outcome of ‘type 2 Diabetes Mellitus and Breast cancer’ association pivots on hypoxia-hyperglycemia axis. Cancer Cell Int 21(1):351
Wei B, Li L, He A, Zhang Y, Sun M, Xu Z (2016) Hippocampal NMDAR-Wnt-catenin signaling disrupted with cognitive deficits in adolescent offspring exposed to prenatal hypoxia. Brain Res 1631:157–164
Biswal S, Das D, Barhwal K, Kumar A, Nag TC, Thakur MK, Hota SK, Kumar B (2017) Epigenetic regulation of SNAP25 prevents Progressive Glutamate Excitotoxicty in hypoxic CA3 neurons. Mol Neurobiol 54(8):6133–6147
Kiouptsi K, Finger S, Garlapati VS, Knorr M, Brandt M, Walter U, Wenzel P, Reinhardt C (2019) : Hypoxia evokes increased PDI and PDIA6 expression in the infarcted myocardium of ex-germ-free and conventionally raised mice. Biology open 8(1)
Malfettone A, Silvestris N, Paradiso A, Mattioli E, Simone G, Mangia A (2012) Overexpression of nuclear NHERF1 in advanced Colorectal cancer: association with hypoxic microenvironment and Tumor invasive phenotype. Exp Mol Pathol 92(3):296–303
Chen Y, Tang M, Xiong J, Gao Q, Cao W, Huang J (2022) GRB10 is a novel oncogene associated with cell proliferation and prognosis in glioma. Cancer Cell Int 22(1):223
Wu K, Mao YY, Chen Q, Zhang B, Zhang S, Wu HJ, Li Y (2021) Hypoxia-induced ROS promotes mitochondrial fission and cisplatin chemosensitivity via HIF-1alpha/Mff regulation in head and neck squamous cell carcinoma. Cell Oncol (Dordr) 44(5):1167–1181
Mu H, Yu G, Li H, Wang M, Cui Y, Zhang T, Song T, Liu C (2021) Mild chronic hypoxia-induced HIF-2alpha interacts with c-MYC through competition with HIF-1alpha to induce hepatocellular carcinoma cell proliferation. Cell Oncol (Dordr) 44(5):1151–1166
Eltzschig HK, Carmeliet P (2011) Hypoxia and inflammation. N Engl J Med 364(7):656–665
Masuda S, Kato K, Ishibashi M, Nishibata Y, Sugimoto A, Nakazawa D, Tanaka S, Tomaru U, Tsujino I, Ishizu A (2022) Phorbol 12-myristate 13-acetate stimulation under hypoxia induces nuclear swelling with DNA outflow but not extracellular trap formation of neutrophils. Exp Mol Pathol 125:104754
Funding
This work was funded by the National Natural Science Foundation of China [82160554(J. Huang), 81860449(X.Xia)], Natural Science Foundation of Hunan Province [2020JJ8051 (J. Huang)], Health Commission of Guangxi [Z20200664(X. Zhou)]
Author information
Authors and Affiliations
Contributions
JH, XWX, and YGT designed the study and wrote the paper. YBC and JLN analyzed the data. YBC and XKZ collected clinical data and performed follow-ups. YBC and JLN performed in vitro experiments. LS and BKY performed the analysis of bioinformatics. LZW, ZLW, and JHH performed in vivo experiments. All authors have contributed to the article and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The data used in this research was used in the case of honoring patient-physician confidentiality, which protected the patient’s privacy and met the ethical requirements and approved by the Research Ethics Committee of the Xiangya hospital.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, Y., Ning, J., Shu, L. et al. CPLX2 is a novel tumor suppressor and improves the prognosis in glioma. J Neurooncol 167, 63–74 (2024). https://doi.org/10.1007/s11060-023-04548-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-023-04548-4