Abstract
Purpose
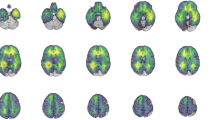
Across several clinical populations, higher white matter hyperintensity (WMH) burden is consistently associated with decreases in cognitive performance, especially processing speed. Research of childhood cancer survivors has not utilized WMH quantification methodology to better understand the impact of WMH burden and its relationship with core cognitive skills. The present study aimed to quantify WMH volumes in a sample of long-term survivors of childhood cerebellar tumor and investigate the relationships with performance on a measure of oral processing speed. To further explore brain–behavior relationships, multivariate sparse canonical correlations was employed to identify WMH areas that predict processing speed performance.
Methods
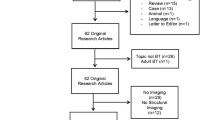
Thirty-five survivors and 56 healthy controls underwent neuroimaging and completed a measure of oral processing speed. The survivor group was further divided based on treatment (i.e., chemoradiation therapy (n = 20) vs. surgery only (n = 15)) to better understand the impact of treatment.
Results
Survivors, and especially those treated with chemoradiation therapy, showed higher total WMH volumes and slower processing speed. Higher total WMH volumes were significantly associated with poorer processing speed (r = − 0.492, p = 0.003). Multivariate brain–behavior relationships revealed that periventricular WMHs were significantly associated with slower processing speed performance (p < 0.05).
Conclusion
Results exemplify that long-term survivors treated with and without chemoradiation therapy are at increased risk of developing higher WMH volumes compared to healthy peers. In addition, processing speed was robustly shown to be related to periventricular WMHs using an automated neuroimaging pipeline. This methodology to monitor WMH burden has the potential to be implemented efficiently with routine clinical neuroimaging of cancer survivors.



Similar content being viewed by others
Data availability
Anonymized datasets are available from the corresponding author on reasonable request.
Code availability
Not applicable.
References
DeCarli C, Fletcher E, Ramey V, Harvey D, Jagust WJ (2005) Anatomical mapping of white matter hyperintensities (wmh) exploring the relationships between periventricular WMH, deep WMH, and total WMH burden. Stroke 36:50–55
Vannorsdall TD, Waldstein SR, Kraut M, Pearlson GD, Schretlen DJ (2009) White matter abnormalities and cognition in a community sample. Arch Clin Neuropsychol 24:209–217
Kloppenborg RP, Nederkoorn PJ, Geerlings MI, van den Berg E (2014) Presence and progression of white matter hyperintensities and cognition: a meta-analysis. Neurology 82:2127–2138
Reinhold H, Calvo W, Hopewell J, Van den Berg A (1990) Development of blood vessel-related radiation damage in the fimbria of the central nervous system. Int J Radiat Oncol* Biol* Phys 18:37–42
Reddick WE, Glass JO, Helton KJ, Langston JW, Xiong X, Wu S, Pui C-H (2005) Prevalence of leukoencephalopathy in children treated for acute lymphoblastic leukemia with high-dose methotrexate. Am J Neuroradiol 26:1263–1269
Briere ME, Scott JG, McNall-Knapp RY, Adams RL (2008) Cognitive outcome in pediatric brain tumor survivors: delayed attention deficit at long-term follow-up. Pediatr Blood Cancer 50:337–340. https://doi.org/10.1002/pbc.21223
Brinkman TM, Reddick WE, Luxton J, Glass JO, Sabin ND, Srivastava DK, Robison LL, Hudson MM, Krull KR (2012) Cerebral white matter integrity and executive function in adult survivors of childhood medulloblastoma. Neuro Oncol 14(Suppl 4):iv25–i36. https://doi.org/10.1093/neuonc/nos214
King TZ, Ailion AS, Fox ME, Hufstetler SM (2019) Neurodevelopmental model of long-term outcomes of adult survivors of childhood brain tumors. Child Neuropsychol 25:1–21
Palmer SL (2008) Neurodevelopmental impact on children treated for medulloblastoma: a review and proposed conceptual model. Dev Disabil Res Rev 14:203–210
Wolfe KR, Madan-Swain A, Kana RK (2012) Executive dysfunction in pediatric posterior fossa tumor survivors: a systematic literature review of neurocognitive deficits and interventions. Dev Neuropsychol 37:153–175. https://doi.org/10.1080/87565641.2011.632462
Semmel ES, Quadri TR, King TZ (2020) Oral processing speed as a key mechanism in the relationship between neurological risk and adaptive functioning in survivors of pediatric brain tumors. Pediatr Blood Cancer 67:e28575
Gunning-Dixon FM, Raz N (2000) The cognitive correlates of white matter abnormalities in normal aging: a quantitative review. Neuropsychology 14:224
Fouladi M, Chintagumpala M, Laningham FH, Ashley D, Kellie SJ, Langston JW, McCluggage CW, Woo S, Kocak M, Krull K (2004) White matter lesions detected by magnetic resonance imaging after radiotherapy and high-dose chemotherapy in children with medulloblastoma or primitive neuroectodermal tumor. J Clin Oncol 22:4551–4560
Lai J-S, Bregman C, Zelko F, Nowinski C, Cella D, Beaumont JJ, Goldman S (2017) Parent-reported cognitive function is associated with leukoencephalopathy in children with brain tumors. Qual Life Res 26:2541–2550
Douw L, Klein M, Fagel SS, van den Heuvel J, Taphoorn MJ, Aaronson NK, Postma TJ, Vandertop WP, Mooij JJ, Boerman RH (2009) Cognitive and radiological effects of radiotherapy in patients with low-grade glioma: long-term follow-up. Lancet Neurol 8:810–818
Pryweller JR, Glass JO, Sabin ND, Laningham FH, Li Y, Jacola LM, Conklin HM, Reddick WE (2021) Characterization of leukoencephalopathy and association with later neurocognitive performance in pediatric acute lymphoblastic leukemia. Investig Radiol 56:117–126
van der Land V, Hijmans CT, de Ruiter M, Mutsaerts HJ, Cnossen MH, Engelen M, Majoie CB, Nederveen AJ, Grootenhuis MA, Fijnvandraat K (2015) Volume of white matter hyperintensities is an independent predictor of intelligence quotient and processing speed in children with sickle cell disease. Br J Haematol 168:553–556. https://doi.org/10.1111/bjh.13179
Duering M, Zieren N, Hervé D, Jouvent E, Reyes S, Peters N, Pachai C, Opherk C, Chabriat H, Dichgans M (2011) Strategic role of frontal white matter tracts in vascular cognitive impairment: a voxel-based lesion-symptom mapping study in CADASIL. Brain 134:2366–2375
Turken U, Whitfield-Gabrieli S, Bammer R, Baldo JV, Dronkers NF, Gabrieli JD (2008) Cognitive processing speed and the structure of white matter pathways: convergent evidence from normal variation and lesion studies. Neuroimage 42:1032–1044
Biesbroek JM, Kuijf HJ, van der Graaf Y, Vincken KL, Postma A, Mali WP, Biessels GJ, Geerlings MI, Group SS (2013) Association between subcortical vascular lesion location and cognition: a voxel-based and tract-based lesion-symptom mapping study. The SMART-MR study. PloS One 8:e60541
First MB, Spitzer RL, Gibbon M, Williams JB (2002) Structured clinical interview for DSM-IV-TR axis I disorders, research version, patient edition (SCID-I/P). Biometrics Research, New York State Psychiatric Institute, New York
Smith A (1982) Symbol digit modalities test. Western Psychological Services, Los Angeles
Gudbjartsson H, Patz S (1995) The Rician distribution of noisy MRI data. Magn Reson Med 34:910–914
Wiest-Daessle N, Prima S, Coupe P, Morrissey SP, Barillot C (2008) Rician noise removal by non-local means filtering for low signal-to-noise ratio MRI: applications to DT-MRI. Lect Notes Comput Sci 5242:171–179
Coupe P, Yger P, Prima S, Hellier P, Kervrann C, Barillot C (2008) An optimized blockwise nonlocal means denoising filter for 3-D magnetic resonance images. IEEE Trans Med Imaging 27:425–441. https://doi.org/10.1109/TMI.2007.906087
Yushkevich PA, Piven J, Hazlett HC, Smith RG, Ho S, Gee JC, Gerig G (2006) User-guided 3D active contour segmentation of anatomical structures: significantly improved efficiency and reliability. Neuroimage 31:1116–1128. https://doi.org/10.1016/j.neuroimage.2006.01.015
Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM (2004) Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 23(Suppl 1):S208–S219. https://doi.org/10.1016/j.neuroimage.2004.07.051
Zhang Y, Brady M, Smith S (2001) Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Trans Med Imaging 20:45–57. https://doi.org/10.1109/42.906424
Lutkenhoff ES, Rosenberg M, Chiang J, Zhang K, Pickard JD, Owen AM, Monti MM (2014) Optimized brain extraction for pathological brains (optiBET). PLoS One 9:e115551. https://doi.org/10.1371/journal.pone.0115551
Pustina D, Coslett HB, Turkeltaub PE, Tustison N, Schwartz MF, Avants B (2016) Automated segmentation of chronic stroke lesions using LINDA: lesion identification with neighborhood data analysis. Human brain mapping 37:1405–1421
Schmidt P, Gaser C, Arsic M, Buck D, Förschler A, Berthele A, Hoshi M, Ilg R, Schmid VJ, Zimmer C (2012) An automated tool for detection of FLAIR-hyperintense white-matter lesions in multiple sclerosis. Neuroimage 59:3774–3783
Jenkinson M, Bannister P, Brady M, Smith S (2002) Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage 17:825–841. https://doi.org/10.1016/s1053-8119(02)91132-8
Jenkinson M, Smith S (2001) A global optimisation method for robust affine registration of brain images. Med Image Anal 5:143–156. https://doi.org/10.1016/s1361-8415(01)00036-6
Yourganov G, Fridriksson J, Stark B, Rorden C (2018) Removal of artifacts from resting-state fMRI data in stroke. NeuroImage Clin 17:297–305
Pustina D, Avants B, Faseyitan OK, Medaglia JD, Coslett HB (2018) Improved accuracy of lesion to symptom mapping with multivariate sparse canonical correlations. Neuropsychologia 115:154–166
Anderson FS, Kunin-Batson AS (2009) Neurocognitive late effects of chemotherapy in children: the past 10 years of research on brain structure and function. Pediatr Blood Cancer 52:159–164. https://doi.org/10.1002/pbc.21700
Ailion AS, King TZ, Roberts SR, Tang B, Turner JA, Conway CM, Crosson B (2020) Double dissociation of auditory attention span and visual attention in long-term survivors of childhood cerebellar tumor: a deterministic tractography study of the cerebellar-frontal and the superior longitudinal fasciculus pathways. J Int Neuropsychol Soc 26:10
King TZ, Wang L, Mao H (2015) Disruption of white matter integrity in adult survivors of childhood brain tumors: correlates with long-term intellectual outcomes. PLoS One 10:e0131744. https://doi.org/10.1371/journal.pone.0131744
Koustenis E, Hernaiz Driever P, de Sonneville L, Rueckriegel SM (2016) Executive function deficits in pediatric cerebellar tumor survivors. Eur J Paediatr Neurol 20:25–37. https://doi.org/10.1016/j.ejpn.2015.11.001
Glass JO, Ogg RJ, Hyun JW, Harreld JH, Schreiber JE, Palmer SL, Li Y, Gajjar AJ, Reddick WE (2017) Disrupted development and integrity of frontal white matter in patients treated for pediatric medulloblastoma. Neuro Oncol 19:1408–1418. https://doi.org/10.1093/neuonc/nox062
Palmer SL, Glass JO, Li Y, Ogg R, Qaddoumi I, Armstrong GT, Wright K, Wetmore C, Broniscer A, Gajjar A, Reddick WE (2012) White matter integrity is associated with cognitive processing in patients treated for a posterior fossa brain tumor. Neuro Oncol 14:1185–1193. https://doi.org/10.1093/neuonc/nos154
Scantlebury N, Bouffet E, Laughlin S, Strother D, McConnell D, Hukin J, Fryer C, Laperriere N, Montour-Proulx I, Keene D, Fleming A, Jabado N, Liu F, Riggs L, Law N, Mabbott DJ (2016) White matter and information processing speed following treatment with cranial-spinal radiation for pediatric brain tumor. Neuropsychology 30:425–438. https://doi.org/10.1037/neu0000258
Bolandzadeh N, Davis JC, Tam R, Handy TC, Liu-Ambrose T (2012) The association between cognitive function and white matter lesion location in older adults: a systematic review. BMC Neurol 12:126
Van den Heuvel D, Ten Dam V, De Craen A, Admiraal-Behloul F, Olofsen H, Bollen E, Jolles J, Murray H, Blauw G, Westendorp R (2006) Increase in periventricular white matter hyperintensities parallels decline in mental processing speed in a non-demented elderly population. J Neurol Neurosurg Psychiatry 77:149–153
Clark SV, Semmel ES, Aleksonis HA, Steinberg SN, King TZ (2021) Cerebellar-subcortical-cortical systems as modulators of cognitive functions. Neuropsychol Rev. https://doi.org/10.1007/s11065-020-09465-1
Greenberger BA, Yock TI (2020) The role of proton therapy in pediatric malignancies: Recent advances and future directions. Seminars in Oncology 47:1. https://doi.org/10.1053/j.seminoncol.2020.02.002
Gondi V, Yock TI, Mehta MP (2016) Proton therapy for paediatric CNS tumours—improving treatment-related outcomes. Nat Rev Neurol 12:334
Dennis M, Yeates KO, Taylor HG, Fletcher JM (2013) Brain reserve capacity, cognitive reserve capacity, and age-based functional plasticity after congenital and acquired brain injury in children. In: Cognitive reserve. Psychology Press, pp 70–100
Edelstein K, Spiegler BJ, Fung S, Panzarella T, Mabbott DJ, Jewitt N, D’Agostino NM, Mason WP, Bouffet E, Tabori U (2011) Early aging in adult survivors of childhood medulloblastoma: long-term neurocognitive, functional, and physical outcomes. Neurooncology 13:536–545
Funding
Supported by the American Cancer Society (#RSGPB-CPPB-114044). HA was supported by a doctoral fellowship provided by the Georgia State University Brains and Behavior Initiative.
Author information
Authors and Affiliations
Contributions
HAA: primary author, conceptualization, statistical analysis, writing-original draft, writing-review, and editing. LCK: methodology, statistical analysis, writing-review, and editing. TZK: senior author, data collection, conceptualization, writing-review, and editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Aleksonis, H.A., Krishnamurthy, L.C. & King, T.Z. White matter hyperintensity volumes are related to processing speed in long-term survivors of childhood cerebellar tumors. J Neurooncol 154, 63–72 (2021). https://doi.org/10.1007/s11060-021-03799-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-021-03799-3