Abstract
Introduction
Label-free Raman-based imaging techniques create the possibility of bringing chemical and histologic data into the operation room. Relying on the intrinsic biochemical properties of tissues to generate image contrast and optical tissue sectioning, Raman-based imaging methods can be used to detect microscopic tumor infiltration and diagnose brain tumor subtypes.
Methods
Here, we review the application of three Raman-based imaging methods to neurosurgical oncology: Raman spectroscopy, coherent anti-Stokes Raman scattering (CARS) microscopy, and stimulated Raman histology (SRH).
Results
Raman spectroscopy allows for chemical characterization of tissue and can differentiate normal and tumor-infiltrated tissue based on variations in macromolecule content, both ex vivo and in vivo. To improve signal-to-noise ratio compared to conventional Raman spectroscopy, a second pulsed excitation laser can be used to coherently drive the vibrational frequency of specific Raman active chemical bonds (i.e. symmetric stretching of –CH2 bonds). Coherent Raman imaging, including CARS and stimulated Raman scattering microscopy, has been shown to detect microscopic brain tumor infiltration in fresh brain tumor specimens with submicron image resolution. Advances in fiber-laser technology have allowed for the development of intraoperative SRH as well as artificial intelligence algorithms to facilitate interpretation of SRH images. With molecular diagnostics becoming an essential part of brain tumor classification, preliminary studies have demonstrated that Raman-based methods can be used to diagnose glioma molecular classes intraoperatively.
Conclusions
These results demonstrate how label-free Raman-based imaging methods can be used to improve the management of brain tumor patients by detecting tumor infiltration, guiding tumor biopsy/resection, and providing images for histopathologic and molecular diagnosis.

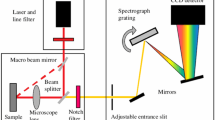
Figure and Caption adapted from Figs. 1 and 3 from [7]

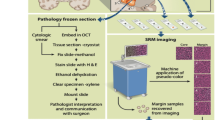
Figure and Caption adapted from Fig. 4 from [26]

Figure and Caption adapted from Fig. 2 from [8]

Figure and Caption adapted from Fig. 3 from [8]

Figure and Caption adapted from Figs. 1 and 3 from [9]
Similar content being viewed by others
References
Senft C, Bink A, Franz K et al (2011) Intraoperative MRI guidance and extent of resection in glioma surgery: a randomised, controlled trial. Lancet Oncol 12:997–1003
Hollon T, Hervey-Jumper SL, Sagher O, Orringer DA (2015) Advances in the surgical management of low-grade glioma. Semin Radiat Oncol 25:181–188
Stummer W, Pichlmeier U, Meinel T et al (2006) Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: a randomised controlled multicentre phase III trial. Lancet Oncol 7:392–401
Lau D, Hervey-Jumper SL, Chang S et al (2016) A prospective Phase II clinical trial of 5-aminolevulinic acid to assess the correlation of intraoperative fluorescence intensity and degree of histologic cellularity during resection of high-grade gliomas. J Neurosurg 124:1300–1309
Hollon T, Lewis S, Freudiger CW et al (2016) Improving the accuracy of brain tumor surgery via Raman-based technology. Neurosurg Focus 40:E9
Kalkanis SN, Kast RE, Rosenblum ML et al (2014) Raman spectroscopy to distinguish grey matter, necrosis, and glioblastoma multiforme in frozen tissue sections. J Neurooncol 116:477–485
Jermyn M, Mok K, Mercier J et al (2015) Intraoperative brain cancer detection with Raman spectroscopy in humans. Sci Transl Med 7:274ra19
Orringer DA, Pandian B, Niknafs YS et al (2017) Rapid intraoperative histology of unprocessed surgical specimens via fibre-laser-based stimulated Raman scattering microscopy. Nat Biomed Eng. https://doi.org/10.1038/s41551-016-0027
Uckermann O, Yao W, Juratli TA et al (2018) IDH1 mutation in human glioma induces chemical alterations that are amenable to optical Raman spectroscopy. J Neurooncol 139:261–268
Raman CV, Krishnan KS (1928) A new type of secondary radiation. Nature 121:501–502
Tashibu K (1990) Analysis of water content in rat brain using Raman spectroscopy. No To Shinkei 42:999–1004
Krafft C, Neudert L, Simat T, Salzer R (2005) Near infrared Raman spectra of human brain lipids. Spectrochim Acta A Mol Biomol Spectrosc 61:1529–1535
Köhler M, Machill S, Salzer R, Krafft C (2009) Characterization of lipid extracts from brain tissue and tumors using Raman spectroscopy and mass spectrometry. Anal Bioanal Chem 393:1513–1520
Kirsch M, Schackert G, Salzer R, Krafft C (2010) Raman spectroscopic imaging for in vivo detection of cerebral brain metastases. Anal Bioanal Chem 398:1707–1713
Koljenović S, Choo-Smith L-P, Bakker Schut TC et al (2002) Discriminating vital tumor from necrotic tissue in human glioblastoma tissue samples by Raman spectroscopy. Lab Invest 82:1265–1277
Kast RE, Auner GW, Rosenblum ML et al (2014) Raman molecular imaging of brain frozen tissue sections. J Neurooncol 120:55–62
Kast R, Auner G, Yurgelevic S et al (2015) Identification of regions of normal grey matter and white matter from pathologic glioblastoma and necrosis in frozen sections using Raman imaging. J Neurooncol 125:287–295
Desroches J, Jermyn M, Mok K et al (2015) Characterization of a Raman spectroscopy probe system for intraoperative brain tissue classification. Biomed Opt Express 6:2380–2397
Jermyn M, Desroches J, Mercier J et al (2016) Neural networks improve brain cancer detection with Raman spectroscopy in the presence of operating room light artifacts. J Biomed Opt 21:94002
Jermyn M, Desroches J, Mercier J et al (2016) Raman spectroscopy detects distant invasive brain cancer cells centimeters beyond MRI capability in humans. Biomed Opt Express 7:5129–5137
Jermyn M, Mercier J, Aubertin K et al (2017) Highly accurate detection of cancer in situ with intraoperative, label-free, multimodal optical spectroscopy. Cancer Res 77:3942–3950
Evans CL, Xu X, Kesari S et al (2007) Chemically-selective imaging of brain structures with CARS microscopy. Opt Express 15:12076–12087
Evans CL, Xie XS (2008) Coherent anti-stokes Raman scattering microscopy: chemical imaging for biology and medicine. Annu Rev Anal Chem 1:883–909
Uckermann O, Galli R, Tamosaityte S et al (2014) Label-free delineation of brain tumors by coherent anti-stokes Raman scattering microscopy in an orthotopic mouse model and human glioblastoma. PLoS ONE 9:e107115
Galli R, Uckermann O, Temme A et al (2017) Assessing the efficacy of coherent anti-stokes Raman scattering microscopy for the detection of infiltrating glioblastoma in fresh brain samples. J Biophotonics 10:404–414
Camp CH Jr, Lee YJ, Heddleston JM et al (2014) High-speed coherent Raman fingerprint imaging of biological tissues. Nat Photonics 8:627–634
Freudiger CW, Min W, Saar BG et al (2008) Label-free biomedical imaging with high sensitivity by stimulated Raman scattering microscopy. Science 322:1857–1861
Saar BG, Freudiger CW, Reichman J et al (2010) Video-rate molecular imaging in vivo with stimulated Raman scattering. Science 330:1368–1370
Ji M, Orringer DA, Freudiger CW et al (2013) Rapid, label-free detection of brain tumors with stimulated Raman scattering microscopy. Sci Transl Med 5:201ra119
Lu F-K, Calligaris D, Olubiyi OI et al (2016) Label-free neurosurgical pathology with stimulated Raman imaging. Cancer Res 76:3451–3462
Ji M, Lewis S, Camelo-Piragua S et al (2015) Detection of human brain tumor infiltration with quantitative stimulated Raman scattering microscopy. Sci Transl Med 7:309ra163
Freudiger CW, Yang W, Holtom GR et al (2014) Stimulated Raman scattering microscopy with a robust fibre laser source. Nat Photonics 8:153–159
Hollon TC, Lewis S, Pandian B et al (2018) Rapid intraoperative diagnosis of pediatric brain tumors using stimulated Raman histology. Cancer Res 78:278–289
Mahe E, Ara S, Bishara M et al (2013) Intraoperative pathology consultation: error, cause and impact. Can J Surg 56:E13–E18
Somerset HL, Kleinschmidt-DeMasters BK (2011) Approach to the intraoperative consultation for neurosurgical specimens. Adv Anat Pathol 18:446–449
Anna I, Bartosz P, Lech P, Halina A (2017) Novel strategies of Raman imaging for brain tumor research. Oncotarget 8:85290–85310
Shi L, Zheng C, Shen Y et al (2018) Optical imaging of metabolic dynamics in animals. Nat Commun 9:2995
Hu F, Lamprecht MR, Wei L et al (2016) Bioorthogonal chemical imaging of metabolic activities in live mammalian hippocampal tissues with stimulated Raman scattering. Sci Rep 6:39660
Fu D, Zhou J, Zhu WS et al (2014) Imaging the intracellular distribution of tyrosine kinase inhibitors in living cells with quantitative hyperspectral stimulated Raman scattering. Nat Chem 6:614–622
Hollon TC, Orringer DA (2018) Shedding light on IDH1 mutation in gliomas. Clin Cancer Res. 24:2467–2469
Zhou Y, Liu C-H, Wu B et al (2019) Optical biopsy identification and grading of gliomas using label-free visible resonance Raman spectroscopy. J Biomed Opt 24:1–12
Livermore LJ, Isabelle M, Bell IM et al (2019) Rapid intraoperative molecular genetic classification of gliomas using Raman spectroscopy. Neuro Oncol Adv. https://doi.org/10.1093/noajnl/vdz008
Uckermann O, Juratli TA, Galli R et al (2018) Optical Analysis of glioma: fourier-transform infrared spectroscopy reveals the IDH1 mutation status. Clin Cancer Res 24:2530–2538
Cancer Genome Atlas Research Network, Brat DJ, Verhaak RGW et al (2015) Comprehensive, integrative genomic analysis of diffuse lower-grade gliomas. N Engl J Med 372:2481–2498
Louis DN, Perry A, Reifenberger G et al (2016) The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol 131:803–820
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Dr. Orringer is a shareholder in Invenio Imaging, Inc.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hollon, T., Orringer, D.A. Label-free brain tumor imaging using Raman-based methods. J Neurooncol 151, 393–402 (2021). https://doi.org/10.1007/s11060-019-03380-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-019-03380-z