Abstract
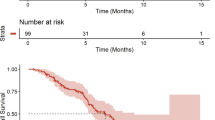
Targeting angiogenesis in glioblastoma (GBM) may improve patient outcome by normalizing tumor vasculature and improving delivery of chemotherapeutics and oxygen. Consequently, concomitant administration of small molecule inhibitors of the VEGF pathway will likely have a positive impact on chemoradiation treatment outcome. We conducted a Phase I study of vatalanib, a small molecule inhibitor of VEGFR, PDGFR, and c-kit in patients with newly diagnosed GBM receiving radiation, temozolomide, and an enzyme-inducing anti-epileptic drug in order to determine the MTD of vatalanib in this patient population. We incorporated circulating biomarker and SNP analyses and pharmacokinetic studies. Nineteen patients were enrolled and the MTD was not reached at the time of study termination. Vatalanib was well tolerated with only 2 DLTs (thrombocytopenia and elevated transaminases). Other grade 3/4 toxicities included leukopenia, lymphopenia, neutropenia, and hand-foot syndrome. There were no wound-healing complications. Of the 13 patients evaluable for a radiographic response, 2 had a partial response and 9 had stable disease. Vatalanib significantly increased PlGF and sVEGFR1 in plasma circulation and decreased sVEGFR2 and sTie2. Plasma collagen IV increased significantly by day 50 of treatment. Vatalanib was well tolerated and this study demonstrates the safety of oral small molecule inhibitors in newly diagnosed GBM patients. Blood biomarkers may be useful as pharmacodynamic markers of response to anti-angiogenic therapies.

Similar content being viewed by others
References
Jain R (2005) Antiangiogenic therapy for cancer: current and emerging concepts. Oncology 19(4 Suppl 3):7–16
Winkler F, Kozin SV, Tong RT, Chae SS, Booth MF, Garkavtsev I, Xu L, Hicklin DJ, Fukumura D, di Tomaso E, Munn LL, Jain RK, Winkler F, Kozin SV, Tong RT, Chae S-S, Booth MF, Garkavtsev I, Xu L, Hicklin DJ, Fukumura D, di Tomaso E, Munn LL, Jain RK (2004) Kinetics of vascular normalization by VEGFR2 blockade governs brain tumor response to radiation: role of oxygenation, angiopoietin-1, and matrix metalloproteinases. Cancer Cell 6(6):553–563
Jain R (2005) Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science 307:58–62
Wood J, Bold G, Buchdunger E, Cozens R, Ferrari S, Frei J, Hofmann F, Mestan J, Mett H, O’Reilly T, Persohn E, Rosel J, Schnell C, Stover D, Theuer A, Towbin H, Wenger F, Woods-Cook K, Menrad A, Siemeister G, Schirner M, Thierauch K, Schneider M, Drevs J, Martiny-Baron G, Totzke F (2000) PTK787/ZK 222584, a novel and potent inhibitor of vascular endothelial growth factor receptor tyrosine kinases, impairs vascular endothelial growth factor-induced responses and tumor growth after oral administration. Cancer Res 60(8):2178–2189
Thomas AL, Morgan B, Horsfield MA, Higginson A, Kay A, Lee L, Masson E, Puccio-Pick M, Laurent D, Steward WP (2005) Phase I study of the safety, tolerability, pharmacokinetics, and pharmacodynamics of PTK787/ZK 222584 administered twice daily in patients with advanced cancer. J Clin Oncol 23(18):4162–4171
Goldbrunner R, Bendszus M, Wood J, Kiderlen M, Sasaki M, Tonn J (2004) PTK787/ZK222584, an inhibitor of vascular endothelial growth factor receptor tyrosine kinases, decreases glioma growth and vascularization. Neurosurgery 55(2):426–432
Conrad C, Friedman H, Reardon D, Provenzale J, Jackson E, Serajuddin H, Laurent D, Chen B, Yung WKA (2004) A phase I/II trial of single-agent PTK 787/ZK 222584 (PTK/ZK), a novel, oral angiogenesis inhibitor, in patients with recurrent glioblastoma multiforme (GBM). ASCO Annual Meeting Proceedings: Abstr 1512
Reardon D, Friedman H, Yung WKA, Brada M, Conrad C, Provenzale J, Jackson E, Serajuddin H, Chen B, Laurent D (2004) A phase I/II trial of PTK787/ZK 222584 (PTK/ZK), a novel, oral angiogenesis inhibitor, in combination with either temozolomide or lomustine for patients with recurrent glioblastoma multiforme (GBM). ASCO Annual Meeting Proceedings: Abstr 1513
Reardon DA, Egorin MJ, Desjardins A, Vredenburgh JJ, Beumer JH, Lagattuta TF, Gururangan S, Herndon JE II, Salvado AJ, Friedman HS (2009) Phase I pharmacokinetic study of the vascular endothelial growth factor receptor tyrosine kinase inhibitor vatalanib (PTK787) plus imatinib and hydroxyurea for malignant glioma. Cancer 115(10):2188–2198. doi:10.1002/cncr.24213
Burstein HJ, Elias AD, Rugo HS, Cobleigh MA, Wolff AC, Eisenberg PD, Lehman M, Adams BJ, Bello CL, DePrimo SE, Baum CM, Miller KD (2008) Phase II study of sunitinib malate, an oral multitargeted tyrosine kinase inhibitor, in patients with metastatic breast cancer previously treated with an anthracycline and a taxane. J Clin Oncol 26(11):1810–1816. doi:JCO.2007.14.5375[pii]10.1200/JCO.2007.14.5375
Drevs J MM, Mross K et al (2005) Phase I clinical evaluation of AZD2171, a highly potent VEGF receptor tyrosine kinase inhibitor, in patients with advanced tumors. J Clin Oncol 23:Abstract 3002
Drevs J, Zirrgiebel U, Schmidt-Gersbach CI, Mross K, Medinger M, Lee L, Pinheiro J, Wood J, Thomas AL, Unger C, Henry A, Steward WP, Laurent D, Lebwohl D, Dugan M, Marme D (2005) Soluble markers for the assessment of biological activity with PTK787/ZK 222584 (PTK/ZK), a vascular endothelial growth factor receptor (VEGFR) tyrosine kinase inhibitor in patients with advanced colorectal cancer from two phase I trials. Ann Oncol 16(4):558–565. doi:mdi118[pii]10.1093/annonc/mdi118
Motzer RJ, Michaelson MD, Redman BG, Hudes GR, Wilding G, Figlin RA, Ginsberg MS, Kim ST, Baum CM, DePrimo SE, Li JZ, Bello CL, Theuer CP, George DJ, Rini BI (2006) Activity of SU11248, a multitargeted inhibitor of vascular endothelial growth factor receptor and platelet-derived growth factor receptor, in patients with metastatic renal cell carcinoma. J Clin Oncol 24(1):16–24. doi:JCO.2005.02.2574[pii]10.1200/JCO.2005.02.2574
Norden-Zfoni A, Desai J, Manola J, Beaudry P, Force J, Maki R, Folkman J, Bello C, Baum C, DePrimo SE, Shalinsky DR, Demetri GD, Heymach JV (2007) Blood-based biomarkers of SU11248 activity and clinical outcome in patients with metastatic imatinib-resistant gastrointestinal stromal tumor. Clin Cancer Res 13(9):2643–2650. doi:13/9/2643[pii]10.1158/1078-0432.CCR-06-0919
Zhu AX, Sahani DV, Duda DG, di Tomaso E, Ancukiewicz M, Catalano OA, Sindhwani V, Blaszkowsky LS, Yoon SS, Lahdenranta J, Bhargava P, Meyerhardt J, Clark JW, Kwak EL, Hezel AF, Miksad R, Abrams TA, Enzinger PC, Fuchs CS, Ryan DP, Jain RK (2009) Efficacy, safety, and potential biomarkers of sunitinib monotherapy in advanced hepatocellular carcinoma: a phase II study. J Clin Oncol 27(18):3027–3035. doi:JCO.2008.20.9908[pii]10.1200/JCO.2008.20.9908
Macdonald DR, Cascino TL, Schold SC Jr, Cairncross JG (1990) Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol 8(7):1277–1280
Duda DG, Cohen KS, Scadden DT, Jain RK, Duda DG, Cohen KS, Scadden DT, Jain RK (2007) A protocol for phenotypic detection and enumeration of circulating endothelial cells and circulating progenitor cells in human blood. Nat Protoc 2(4):805–810
Sorensen AG, Batchelor TT, Zhang WT, Chen PJ, Yeo P, Wang M, Jennings D, Wen PY, Lahdenranta J, Ancukiewicz M, di Tomaso E, Duda DG, Jain RK (2009) A “Vascular normalization index” As potential mechanistic biomarker to predict survival after a single dose of cediranib in recurrent glioblastoma patients. Cancer Res 69(13):5296–5300. doi:0008-5472.CAN-09-0814[pii]10.1158/0008-5472.CAN-09-0814
Brandes AA, Stupp R, Hau P, Lacombe D, Gorlia T, Tosoni A, Mirimanoff RO, Kros JM, van den Bent MJ (2009) Eortc study 26041–22041: Phase I/II study on concomitant and adjuvant temozolomide (TMZ) and radiotherapy (RT) with PTK787/ZK222584 (PTK/ZK) in newly diagnosed glioblastoma. Eur J Cancer 46(2):348–354. doi:S0959-8049(09)00816-8[pii]10.1016/j.ejca.2009.10.029
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gerstner, E.R., Eichler, A.F., Plotkin, S.R. et al. Phase I trial with biomarker studies of vatalanib (PTK787) in patients with newly diagnosed glioblastoma treated with enzyme inducing anti-epileptic drugs and standard radiation and temozolomide. J Neurooncol 103, 325–332 (2011). https://doi.org/10.1007/s11060-010-0390-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-010-0390-7