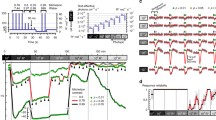
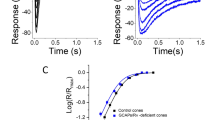
The GPCR signal cascade in retinal photoreceptor cells – rods and cones – supports powerful signal amplification and allows rods to respond reliably to single light quanta. Rods remain functional at light fluxes down to 105 quanta/sec, which is supported by the highly effective light adaptation system. Light adaptation is based on negative feedback circuits, mostly via changes in intracellular Ca2+ ion concentrations. Three calcium regulation loops have been reliably identified – acceleration of quenching of light-activated rhodopsin, acceleration of cGMP synthesis by guanylate cyclase, and increased affinity of cGMP-controlled ion cannels for nucleotide. However, one or two further highly effective mechanisms of adaptation are known to operate, one of which regulates the lifetime of activated phosphodiesterase; for others, the target of regulation is unknown. The mediators of this regulation are also unknown. Our studies of these mechanisms revealed a novel phenomenon, which has been missed in previous studies. We found that recovery of the dark current of rods after cessation of non-saturating adapting light can take 20–30 sec. Furthermore, after formal return of the membrane current to the dark level, cell sensitivity to test stimuli remains decreased for a further 1–2 min. We termed this phenomenon “adaptation memory.” Adaptation memory is reminiscent of the phenomenology of afterimages. The gradual return of the membrane current to the dark level may correspond to fading of the positive afterimage. Prolonged decreases in photoreceptor sensitivity to additional stimulation can create a negative afterimage. As far as we know, this is the first experimental physiological demonstration of the ability to generate afterimages at the level of single photoreceptors.
Similar content being viewed by others
References
E. N. Pugh, Jr., N. Edward, and T. D. Lamb, “Phototransduction in vertebrate rods and cones: molecular mechanisms of amplification, recovery and light adaptation,” in: Handbook of Biological Physics, North-Holland (2000), Vol. 3, pp. 183–255.
V. Y. Arshavsky, T. D. Lamb, and E. N. Pugh, Jr., “G proteins and phototransduction,” Annu. Rev. Physiol., 64, 153–187 (2002).
M. L. Firsov, and V. I. Govardovskii, “Light adaptation of photoreceptors: meaning and mechanisms,” Sens. Sistemy, 15, No. 2, 101–113 (2001).
V. Y. Arshavsky and M. E. Burns, “Photoreceptor signaling: supporting vision across a wide range of light intensities,” J. Biol. Chem., 287, No. 3, 1620–1626 (2012).
J. Chen, M. L. Woodruff, T. Wang, et al., “Channel modulation and the mechanism of light adaptation in mouse rods,” J. Neurosci., 30, No. 48, 16232–16240 (2010).
F. Vinberg, J. Chen, and V. J. Kefalov, “Regulation of calcium homeostasis in the outer segments of rod and cone photoreceptors,” Prog. Retin. Eye Res., 67, 87–101 (2018).
P. D. Calvert, V. I. Govardovskii, V. Y. Arshavsky, and C. L. Makino, “Two temporal phases of light adaptation in retinal rods,” J. Gen. Physiol., 119, No. 2, 129–146 (2002).
L. A. Astakhova, M. L. Firsov, and V. I. Govardovski, “Kinetics of turn-offs of frog rod phototransduction cascade,” J. Gen. Physiol., 132, No. 5, 587–604 (2008).
D. A. Baylor, T. D. Lamb, K.-W. Yau, “The membrane current of single rod outer segments,” J. Physiol., 288, 589–611 (1979).
M. L. Firsov, A. V. Kolesnikov, E. Y. Golobokova, and V. I. Govardovskii, “Two realms of dark adaptation,” Vision Res., 45, No. 2, 147–151 (2005).
R. D. Hamer, S. C. Nicholas, D. Tranchina, et al., “Toward a unifi ed model of vertebrate rod phototransduction,” Vis. Neurosci., 22, 417–436 (2005).
B. M. Invergo, D. Dell’Orco, L. Montanucci, et al., “A comprehensive model of the phototransduction cascade in mouse rod cells,” Mol. Biosystems, 10, No. 6, 1481–1489 (2014).
D. G. Kuz’min, S. V. Travnikov, M. L. Firsov, and V. I. D. G. Govardovskii, “Mathematical modeling of phototransduction and light adaptation in frog retinal rods,” Sens. Sistemy, 18, No. 4, 305–316 (2004).
A. Morshedian and G. L. Fain, “Molecular mechanism of adaptation in vertebrate rods,” in: Vertebrate Photoreceptors, Springer, Tokyo (2014), pp. 73–90.
K. J. W. Craik, “Origin of visual after-images,” Nature, 145, 512 (1940).
R. van Lier, M. Vergeer, and S. Anstis, “Filling-in afterimage colors between the lines,” Curr. Biol., 19, No. 8, R323–R324 (2009).
G. Powell, A. Bompas, and P. Sumner, “Making the incredible credible: Afterimages are modulated by contextual edges more than real stimuli,” J. Vis., 12, No. 10, 1–13 (2012).
S. Zeki, S. Cheadle, J. Pepper, and D. Mylonas, “The constancy of colored after-images,” Front. Hum. Neurosci., 11, 229 (2017).
J. M. Loomis, “The photopigment bleaching hypothesis of complementary after-images,” Vision Res., 12, 1587–1594 (1972).
V. Virsu and P. Laurinen, “Long-lasting afterimages caused by neural adaptation,” Vision Res., 17, No. 7, 853–860 (1977).
G. S. Brindley, “The discrimination of after-images,” J. Physiol., 147, 194–203 (1959).
H. B. Barlow and J. M. B. Sparrock, “The role of afterimages in dark adaptation,” Science, 144, 1309–1314 (1964).
D. I. A. MacLeod, “Rod origin of prolonged afterimages,” Science, 185, 1171–1172 (1974).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Rossiiskii Fiziologicheskii Zhurnal imeni I. M. Sechenova, Vol. 106, No. 4, pp. 462–473, April, 2020.
Rights and permissions
About this article
Cite this article
Rotov, A.Y., Astakhova, L.A., Firsov, M.L. et al. Light Adaptation of Retinal Rods, Adaptation Memory, and Afterimages. Neurosci Behav Physi 51, 116–122 (2021). https://doi.org/10.1007/s11055-020-01046-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11055-020-01046-2