Abstract
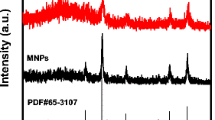
This paper reported an immobilization of Candida rugosa lipase (CRL) onto PAMAM-dendrimer-grafted magnetic nanoparticles synthesized by a modified solvothermal reduction method. The dendritic magnetic nanoparticles were amply characterized by several instrumental measurements, and the CRL was covalently anchored on the three generation supports with glutaraldehyde as coupling reagent. The amount of immobilized enzyme was up to 150 mg/g support and the factors related with the enzyme activity were investigated. The immobilization of lipase improved their performance in wider ranges of pH and temperature. The immobilized lipase exhibited excellent thermal stability and reusability in comparison with free enzyme and can be reused 10 cycles with the enzymatic activity remained above 90 %. The properties of lipase improved obviously after being immobilized on the dendritic supports. The inactive immobilized lipase could be regenerated with glutaraldehyde and Cu2+, respectively. This synthetic strategy was facile and eco-friendly for applications in lipase immobilization.













Similar content being viewed by others
References
Bagheri M, Rodríguez H, Swatloski RP, Spear SK, Daly DT, Rogers RD (2008) Ionic liquid-based preparation of cellulose-dendrimer films as solid supports for enzyme immobilization. Biomacromolecules 9:381–387
Barbosa O, Torres R, Ortiz C, Berenguer-Murcia A, Rodrigues RC, Fernandez-Lafuente R (2013) Heterofunctional supports in enzyme immobilization: from traditional immobilization protocols to opportunities in tuning enzyme properties. Biomacromolecules 14:2433–2462
Barbosa O, Ortiz C, Berenguer-Murcia A, Torres R, Rodrigues RC, Fernandez-Lafuente R (2014) Glutaraldehyde in bio-catalysts design: a useful crosslinker and a versatile tool in enzyme immobilization. RSC Adv 4:1583–1600
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Brady D, Jordaan J (2009) Advances in enzyme immobilization. Biotechnol Lett 31:1639–1650
Bronstein LM, Shifrina ZB (2009) Nanoparticles in dendrimers: from synthesis to application. Nanotechnol Russ 4:576–608
Choudhury AK, Kitaoka M, Hayashi K (2003) Synthesis of a cellobiosylated dimer and trimer and of cellobiose-coated polyamidoamine (PAMAM) dendrimers to study accessibility of an enzyme, cellodextrin phosphorylase. Eur J Org Chem 2003:2462–2470
Crespilho FN, Ghica ME, Florescu M, Nart FC, Oliveira ON, Brett CM (2006) A strategy for enzyme immobilization on layer-by-layer dendrimer-gold nanoparticle electrocatalytic membrane incorporating redox mediator. Electrochem Commun 8:1665–1670
Crooks RM, Zhao M, Sun L, Chechik V, Yeung LK (2001) Dendrimer-encapsulated metal nanoparticles: synthesis, characterization, and applications to catalysis. Acc Chem Res 34:181–190
Demircioǧlu H, Beyenal H, Tanyolaç A, Hasirci N (1994) Immobilization of urease and estimation of effective diffusion coefficients of urea in HEMA and VP copolymer matrices. Polym Int 35:321–327
Deng H, Li XL, Peng Q, Wang X, Chen JP, Li YD (2005) Soft nanotechnology with soft nanoparticles. Angew Chem Int Ed 44:2782
Garcia J, Zhang Y, Taylor H, Cespedes O, Webb ME, Zhou DJ (2011) Multilayer enzyme-coupled magnetic nanoparticles as efficient, reusable biocatalysts and biosensors. Nanoscale 3:3721–3730
Gardossi L, Bianchi D, Klibanov AM (1991) Selective acylation of peptides catalyzed by lipases in organic solvents. J Am Chem Soc 113:6328–6329
Grayson SM, Frechet JMJ (2001) Convergent dendrons and dendrimers: from synthesis to applications. Chem Rev 101:3819–3868
Gupta MN, Kaloti M, Kapoor M, Solanki K (2011) Nanomaterials as matrices for enzyme immobilization. Artif Cells Blood Substit Biotechnol 39:98–109
Hartmann M, Jung D (2010) Biocatalysis with enzymes immobilized on mesoporous hosts: the status quo and future trends. J Mater Chem 20:844–857
Hong J, Xu D, Gong P, Sun H, Dong L, Yao S (2007) Covalent binding of α-chymotrypsin on the magnetic nanogels covered by amino groups. J Mol Catal B Enzym 45:84–90
Hou C, Zhu H, Wu D, Li Y, Hou K, Jiang Y, Li Y (2014) Immobilized lipase on macroporous polystyrene modified by PAMAM-dendrimer and their enzymatic hydrolysis. Process Biochem 49:244–249
Jaeger KE, Reetz MT (1998) Microbial lipases form versatile tools for biotechnology. Trends Biotechnol 16:396–403
Jiang DS, Long SY, Huang J, Xiao HY, Zhou JY (2005) Immobilization of Pycnoporus sanguineus laccase on magnetic chitosan microspheres. Biochem Eng J 25:15–23
Kunzmann A, Andersson B, Thurnherr T, Krug H, Scheynius A, Fadeel B, Scheyniusb A, Fadeel B et al (2011) Toxicology of engineered nanomaterials: focus on biocompatibility, biodistribution and biodegradation. BBA 1810:361–373
Li X, Zhu H, Feng J, Zhang J, Deng X, Zhou B,…, Peng Y (2013) One-pot polylol synthesis of graphene decorated with size-and density-tunable Fe3O4 nanoparticles for porcine pancreatic lipase immobilization. Carbon 60:488–497
Mateo C, Palomo JM, Fernandez-Lorente G, Guisan JM, Fernandez-Lafuente R (2007) Improvement of enzyme activity, stability and selectivity via immobilization techniques. Enzym Microb Technol 40:1451–1463
Migneault I, Dartiguenave C, Bertrand MJ, Waldron KC (2004) Glutaraldehyde: behavior in aqueous solution, reaction with proteins, and application to enzyme crosslinking. Biotechniques 37:790–806
Monsan P (1978) Optimization of glutaraldehyde activation of a support for enzyme immobilization. J Mol Catal A 3:371–384
Narayanan VV, Newkome GR (1998) Supramolecular chemistry within dendritic structures. Top Curr Chem 197:19–77
Omprakash Y, Toyoko I (2005) Covalent-bonded immobilization of lipase on poly (phenylene sulfide) dendrimers and their hydrolysis ability. Biomacromolecules 6:2809–2814
Pahujani S, Kanwar S, Chauhan G, Gupta R (2008) Glutaraldehyde activation of polymer Nylon-6 for lipase immobilization: enzyme characteristics and stability. Bioresour Technol 99:2566–2570
Pfaff A, Müller AH (2011) Hyperbranched glycopolymer grafted microspheres. Macromolecules 44:1266–1272
Rodrigues RC, Ortiz C, Berenguer-Murcia A, Torres R, Fernández-Lafuente R (2013) Modifying enzyme activity and selectivity by immobilization. Chem Soc Rev 42:6290–6307
Schlüter AD, Rabe JP (2000) Dendronized polymers: synthesis, characterization, assembly at interfaces, and manipulation. Angew Chem Int Ed 39:864–883
Tomalia DA, Baker H, Dewald J, Hall M, Kallos G, Martin S, Smith P (1985) A new class of polymers: starburst-dendritic macromolecules. Polym J 17:117–132
Uzun K, Çevik E, Şenel M, Sözeri H, Baykal A, Abasıyanık MF, Toprak MS (2010) Covalent immobilization of invertase on PAMAM-dendrimer modified superparamagnetic iron oxide nanoparticles. J Nanopart Res 12:3057–3067
Vertegel AA, Siegel RW, Dordick JS (2004) Silica nanoparticle size influences the structure and enzymatic activity of adsorbed lysozyme. Langmuir 20:6800–6807
Wang P (2006) Nanoscale biocatalyst systems. Curr Opin Biotechnol 17:574–579
Wang Y, Caruso F (2005) Mesoporous silica spheres as supports for enzyme immobilization and encapsulation. J Chem Mater 17:953–961
Wang S, Su P, Ding F, Yang Y (2013) Immobilization of ellulose on polyamidoamine dendrimer-grafted silica. J Mol Catal B Enzym 89:35–40
Watanabe N, Ota Y, Minoda Y, Yamada K (1977) Isolation and identification of alkaline lipase producing microorganisms, cultural conditions and some properties of crude enzymes. Agric Biol Chem 41:1353–1358
Zhao G, Wang J, Li Y, Chen X, Liu Y (2011) Enzymes immobilized on superparamagnetic Fe3O4@ clays nanocomposites: preparation, characterization, and a new strategy for the regeneration of supports. J Phys Chem C 115:6350–6359
Zhong T, Ai P, Zhou J (2011) Structures and properties of PAMAM dendrimer: a multi-scale simulation study. Fluid Phase Equilib 302:43–47
Zhu H, Hou C, Li Y, Zhao G, Liu X, Hou K, Li Y (2013) One-pot solvothermal synthesis of highly water-dispersible size-tunable functionalized magnetite nanocrystal clusters for lipase immobilization. Chem Asian J 8:1447–1454
Zucca P, Sanjust E (2014) Inorganic materials as supports for covalent enzyme immobilization: methods and mechanisms. Molecules 19:14139–14194
Acknowledgments
The authors thank the financial supports from the National Natural Science Foundation of China (Nos. 21374045, 21074049) and the Fundamental Research Funds for the Central Universities (No. lzujbky-2014-186).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhu, W., Zhang, Y., Hou, C. et al. Covalent immobilization of lipases on monodisperse magnetic microspheres modified with PAMAM-dendrimer. J Nanopart Res 18, 32 (2016). https://doi.org/10.1007/s11051-016-3337-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-016-3337-x