Abstract
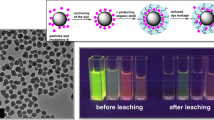
The enzymatic hydrolysis of quinizarin diester in silica nanoparticle (NP) of 200 nm diameter is investigated by confocal fluorescence microscopy. The quinizarin diester substrate and the intermediate quinizarin monoester are non-fluorescent species and only the end product—quinizarin formed by enzymatic hydrolysis produces intense fluorescence of the silica NP. The enzyme activity of lipase adsorbed into silica NP was similar to that observed for lipase chemically bound to silica surface. In both situations, partial aggregation of the silica NP dispersed in thin film of polyvinylpyrrolidone was observed from fluorescence and scanning electron microscopy images. The fluorescence decay of the end product—quinizarin in silica NP was biexponential with decay times of 0.49 and 2.17 ns. These two decay times found are ascribed to quinizarin adsorbed in silica NP and dispersed in the surrounding medium, respectively.









Similar content being viewed by others
References
Ahn K-D, Yoo K-W, Soh J-H, Kang J-H (2009) Fluorescent photoimaging with polymers having protected quinizarin dye precursors by a dry process based on chemical amplification. React Funct Polym 69:111–116
Al-Suhair S (2005) Production of biodiesel by lipase-catalyzed transesterification of vegetable oils: a kinetics study. Biotechnol Prog 21:1442–1448
Bernede JC, Ben Nasrallah T, Jamali M, Mevellec JY, Rabiller C, Proutiere A (1995) Is a hydrogen bound responsible for the optical properties of some dihydroxyanthraquinones: quinizarin and anthraflavic? J Phys Chem Sol 56:1239–1251
Canton G, Riccò R, Marinello F, Carmignato S, Enrichi F (2011) Modified Stöber synthesis of highly luminescent dye-doped silica nanoparticles. J Nanopart Res 13:4349–4356
Duque M, Graupner M, Stutz H, Wicher I, Zechner R, Paltauf F, Hermetter A (1996) New fluorogenic triacylglycerol analogs as substrates for the determination and chiral discrimination of lipase activities. J Lipid Res 37:868–876
Ferreira APG, Frederice R, Janssen KPF, Gehlen MH (2011) Dually fluorescent silica nanoparticles. J Lumin 131:888–893
Gamba M, Lapis AAM, Dupont J (2008) Supported ionic liquid enzymatic catalysis for the production of biodiesel. Adv Synth Catal 350:160–164
Haugland RP, Johnson ID (1993) Detecting enzymes in living cells using fluorogenic substrates. J Fluoresc 3:119–127
Kim J-M, Kang J-H, Han D-K, Lee C-W, Ahn K-D (1998) A novel precursor for color and fluorescence imaging. Chem Mater 10:2332–2334
Liu Q, DeShong P, Zachariah MR (2012) One-sep synthesis of dye-incorporated porous silica particles. J Nanopart Res 14:923
Mateo C, Palomo JM, Fernndez-Lorente G, Guisan JM, Fernandez-Lafuente R (2007) Improvement of enzyme activity, stability and selectivity via immoblization techniques. Enzyme Microb Technol 40:1451–1463
Pal H, Palit DK, Mukherjee T, Mittal JP (1990) Interaction of the excited singlet state of disubstituted anthraquinones with aromatic hydrocarbons: a fluorescence-quenching study. Chem Phys Lett 173:354–359
Palit DK, Pal H, Mukherjee T, Mittal JPJ (1990) Photodynamics of the S1 state of some hydroxy- and amino-substituted naphthoquinones and anthraquinones. J Chem Soc Faraday Trans 86:3861–3869
Pavlidis IV, Vorhaben T, Gournis D, Papadopoulos GK, Bornscheuer UT, Stamatis H (2012) Regulation of catalytic behaviour of hydrolases through interaction with functionalized carbon-based nanomaterials. J Nanopart Res 14:842
Popat A, Hartono SB, Stahr F, Liu J, Qiao SZ, Lu GQ (2011) Mesoporous silica nanoparticles for bioadsorption, enzyme immobilisation, and delivery carries. Nanoscale 3:2801–2818
Samuel J, Raccurt O, Poncelet O, Auge A, Ling WL, Cherns P, Grunwald D, Tillement O (2010) Surface characterization of fluorescent-functionalized silica nanoparticles: from the macroscatle to the nanoscale. J Nanopart Res 12:2255–2265
Shi Q, Yang D, Su Y, Li J, Jiang Z, Jiang Y, Yuan W (2007) Covalent functionalization of multi-walled carbon nanotubes by lipase. J Nanopart Res 9:1205–1210
Stöber W, Fink A (1968) Controlled growth of monodisperse silica spheres in the micron size range. J Colloid Interface Sci 26:62–69
Villeneuve P, Muderhwa JM, Graille J, Haas MJ (2000) Customizing lipases for biocatalysis: a survey of chemical, physical and molecular biological approaches. J Mol Catal B 9:113–148
Yang Y, Babiak P, Reymond J-L (2006) New monofunctionalized fluorescein derivatives for the efficient high-throughput screening of lipases and esterases in aqueous media. Helv Chim Acta 89:404–415
Yo H, Pak J (2013) Synthesis of highly fluorescent silica nanoparticles in a reverse microemulsion through double-layered doping of organic fluorophores. J Nanopart Res 15:1609
Acknowledgments
The authors thank FAPESP and CNPq for financial support of this work. C. A. S. thanks CAPES for a fellowship. This work is also within the scope of INCT-Catálise Research Center in Brazil. MHG thanks Professor Dr. J. Hofkens and Dr. K. P. F. Janssen for the use of the image acquisition and analysis SIS software from the Division of Molecular and Nano Materials of the Katholieke Universiteit Leuven.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sabatini, C.A., Gehlen, M.H. Enzymatic hydrolysis of quinizarin diester by lipase in silica nanoparticles investigated by fluorescence microscopy. J Nanopart Res 16, 2093 (2014). https://doi.org/10.1007/s11051-013-2093-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-013-2093-4