Abstract
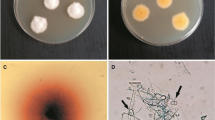
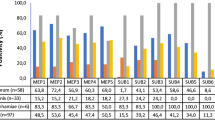
Microsporum canis is a zoophilic dermatophyte and the most common fungus isolated from dogs and cats worldwide. To invade skin, this pathogen uses different enzymes, which may be associated with virulence, that contribute to the fungal pathogenicity. The aim of this study is to compare the expression of enzymes that may be associated with virulence, and thermotolerance of M. canis strains isolated from dogs, cats, and humans. The in vitro expression of the enzymes keratinase, catalase, urease, hemolysin, and aspartic protease was evaluated in 52 M. canis strains recently isolated from 14 human patients, 12 dogs, 15 symptomatic, and 11 asymptomatic cats. In addition, thermotolerance was assessed by comparative analysis of fungal growth at 25 °C and 35 °C. Keratinase activity was low in 34 and moderate in 18 strains. Aspartic-protease activity was low in 7, moderate in 33, and high in 12 strains. Hemolysin activity was low in 44 and moderate in 8 strains. All strains were classified as low producers of catalase. All but three strains produced urease in vitro, with a broad range of activity. The strains presented in vitro growth at the two studied temperatures were classified as presenting low (36.5%), medium (44.3%), or high (19.2%) thermotolerance. There was no statistically significant difference in the new putative virulence-associated factors studied among the different hosts, which suggests that they may have a similar role on human, cat, and dog infection. Also, no difference was observed between strains isolated from symptomatic and asymptomatic cats. This suggests that these factors have a limited impact on the fate of feline dermatophytosis caused by M. canis.



Similar content being viewed by others
Availability of Data and Material
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
de Hoog GS, Dukik K, Monod M, Packeu A, Stubbe D, Hendrick M, Kupsch C, Stielow JB, Freeke J, Goker M, Rezaei-Matehkolaei A, Mirhendi H, Graser Y. Toward a novel multilocus phylogenetic taxonomy for the dermatophytes. Mycopathologia. 2017;182:5–31.
Balda AC, Larsson CE, Otsuka M, Gambale W. Estudo Retrospectivo de Casuística das Dermatofitoses em Cães e Gatos Atendidos no Serviço de Dermatologia da Faculdade de Medicina Veterinária e Zootecnia da Universidade de São Paulo. Acta Sci Vet. 2004;32:133–40.
Elavarashi E, Kindo AJ, Rangarajan S. Enzymatic and nonenzymatic virulence activities of dermatophytes on solid media. J Clin Diagn Res. 2017;11:23–5.
Sharifzadeh A, Shokri H, Khosravi AR. In vitro evaluation of antifungal susceptibility and keratinase, elastase, lipase and DNase activities of different dermatophyte species isolated from clinical specimens in Iran. Mycoses. 2016;59:710–9.
Gnat S, Lagowski D, Nowakiewicz A, Zieba P. Phenotypic characterization of enzymatic activity of clinical dermatophyte isolates from animals with and without skin lesions and humans. J Appl Microbiol. 2018;125:700–9.
Chinnapun D. Virulence factors involved in pathogenicity of dermatophytes. Walailak J Sci Technol. 2015;12:573–9.
Cox GM, Mukherjee J, Cole GT, Casadevall A, Perfect JR. Urease as a virulence factor in experimental cryptococcosis. Infect Immun. 2000;68:443–8.
Kane J, Fisher JB. The differentiation of T. rubrum and T. mentagrophytes by use of Christensen`s urea broth. Can J Microbiol. 1971;17:911–3.
Torres MELM, Herculano PN, Lima MLF, Soares PT, Siqueira ABS, Souza-Motta CM, Porto ALF, Nascimento CO. Isolamento e perfil enzimático de cães e gatos com dermatofitose atendidos em hospitais veterinários do Recife, Pernambuco. Pesq Vet Bras. 2018;38:930–4.
Pichova I, Pavlickova L, Dostal J, Dolejsi E, Hruskova- Heidingsfeldová O, Weber J, Ruml T, Soucek M. Secreted aspartic proteases of Candida albicans, Candida tropicalis, Candida prapsilosis and Candida lusitaniae. Inhibition with peptidomimetic inhibitors. Eur J Biochem. 2001;268:2669–777.
Hansberg W, Salas-Lizana R, Domínguez L. Fungal catalases: function, phylogenetic origin and structure. Arch Biochem Biophys. 2012;525:170–80.
Nevitt T, Thiele DJ. Host iron withholding demands siderophore utilization for Candida glabrata to survive macrophage killing. PLoS Pathog. 2011;7:1–15.
Mesa-Arango AC, Reyes-Montes MDR, Pérez-Mejia A, Navarro-Barranco H, Souza V, Zúniga G, Toriello C. Phenotyping and genotyping of Sporothrix schenckii isolates according to geographic origin and clinical form of Sporotrichosis. J Clin Microbiol. 2002;40:3004–111.
Boechat JS, Oliveira MME, Almeida-Paes R, Gremiao IDF, Machado ACS, Oliveira RVC, Figueiredo ABF, Rabello VBS, Silva KBL, Zancopé-Oliveira RM, Schubach TMP, Pereira SA. Feline sporotrichosis: associations between clinical-epidemiological profiles and phenotypic-genotypic characteristics of the etiological agents in the Rio de Janeiro epizootic area. Mem Inst Oswaldo Cruz. 2018;3:185–96.
Cafarchia C, Figueredo LA, Coccioli C, Camarda A, Otranto D. Enzymatic activity of Microsporum canis and Trichophyton mentagrophytes from breeding rabbits with and without skin lesions. Mycoses. 2011;55:45–9.
Abi-Chacra EA, Souza LO, Cruz LP, Braga-Silva LA, Gonçalves DS, Sodré CL, Ribeiro MD, Seabra SH, Figueiredo-Carvalho MH, Barbedo LS, Zancopé-Oliveira RM, Ziccardi M, Santos AL. Phenotypical properties associated with virulence from clinical isolates belonging to the Candida parapsilosis complex. FEMS Yeast Res. 2013;13:831–48.
Aktas E, Yigit N. Hemolytic activity of dermatophytes species isolated from clinical specimens. J Mycol Med. 2015;25:25–30.
Price MF, Wilkison ID, Gentry LO. Plate method for detection of phospholipase activity in Candida albicans. Sabouraudia. 1982;20:7–14.
Figueiredo-Carvalho MHG, Ramos LS, Barbedo LS, Oliveira JCA, Santos ALS, Almeida-Paes R, Zancopé-Oliveira RM. Relationship between the antifungal susceptibility profile and the production of virulence-related hydrolytic enzymes in Brazilian clinical strains of Candida glabrata. Mediat Inflamm. 2017;2017:1–10.
Almeida-Paes R, Oliveira LC, Oliveira MME, Gutierrez-Galhardo MC, Nosanchuk JD, Zancopé-Oliveira RM. Phenotypic characteristics associated with virulence of clinical isolates from the Sporothrix complex. Biomed Res Int. 2015;2015:212308.
Babicki S, Arndt D, Marcu A, Liang Y, Grant JR, Maciejewski A, Wishart DS. Heatmapper: web-enabled heat mapping for all. Nucleic Acids Res. 2016;44:147–53.
Viani FC, Santos JI, Paula CR, Larson CE, Gambale W. Production of extracellular enzymes by Microsporum canis and their role in its virulence. Med Mycol. 2001;39:463–8.
Viani FC, Viani PRC, Riviera ING, Silva EG, Paula CR, Gambale W. Extracellular proteolytic activity and molecular analysis of Microsporum canis strains isolated from symptomatic and asymptomatic cats. Rev Iberoam Micol. 2007;24:19–23.
Franzot SP, Mukherjee J, Cherniak R, Chen LC, Hamdan JS, Casadevall A. Microevolution of a standard strain of Cryptococcus neoformans resulting in differences in virulence and other phenotypes. Infect Immun. 1998;66:89–97.
Refsnider JM, Poorten TJ, Langhammer PF, Burrowes PA, Rosenblum EB. Genomic correlates of virulence attenuation in the deadly amphibian chytrid fungus, Batrachochytrium dendrobatidis. G3 (Bethesda). 2015;5:2291–8.
Bagut ET, Baldo A, Mathy A, Cambier L, Antoine N, Cozma V, Mignon B. Sub 3 is involved in adherence of Microsporum canis to human and animal epidermis. Vet Microbiol. 2012;160:413–9.
Monod M, Capoccia S, Léchenne B, Zaugg C, Holdom M, Jousson O. Secreted proteases from pathogenic fungi. Int J Med Microbiol. 2002;292:405–19.
Mignon B, Swinnen M, Bouchara JP, Hofinger M, Nikkels A, Pierard G, Gerday C, Losson B. Purification and characterization of a 31.5 KDa keratinolytic subtilisin-like serine protease from Microsporum canis and evidence of its secretion in naturally infected cats. Med Mycol. 1998;36:395–404.
Mathy A, Baldo A, Schoofs L, Cambier L, Defaweux V, Tabart J, Maréchal F, Symoens F, Mignon B. Fungalysin and dipeptidyl-peptidase gene transcription in Microsporum canis strains isolated from symptomatic and asymptomatic cats. Vet Microbiol. 2010;146:179–82.
Nakamura K, Kanno T, Mokudai T, Iwasawa A, Niwano Y, Kohno M. Microbial resistance in relation to catalase activity to oxidative stress induced by photolysis of hydrogen peroxide. Microbiol Immunol. 2012;56:48–55.
Boer M, Duchnik E, Maleszka R, Marchlewicz M. Structural and biophysical characteristics of human skin in maintaining proper epidermal barrier function. Postepy Dermatol Alergol. 2016;33:1–5.
Goic JB, Reineke EL, Drobatz KJ. Comparison of rectal and axillary temperatures in dogs and cats. J Am Vet Med Assoc. 2014;244:1170–5.
Giddey K, Monod M, Barblan J, Potts A, Waridel P, Zaugg C, Quadroni M. Comprehensive analysis of proteins secreted by Trichophyton rubrum and Trichophyton violaceum under in vitro conditions. J Proteome Res. 2007;6:3081–92.
Martins MP, Rossi A, Sanches PR, Bortolossi JC, Martinez-Rossi NM. Comprehensive analysis of the dermatophyte Trichophyton rubrum transcriptional profile reveals dynamic metabolic modulation. Biochem J. 2020;477:873–85.
Tigga RA, Das S, Bhattacharya SN, Saha R, Pandhi D, Datt S, Rai G. Burden of chronic dermatophytosis in a tertiary care hospital: interaction of fungal virulence and host immunity. Mycopathologia. 2018;183(6):951–9.
Acknowledgements
Authors thank Levi G. Cleare for his editorial assistance.
Funding
RMZ-O was supported in part by Conselho Nacional de Desenvolvimento Científico e Tecnológico [CNPq 302796/2017-7] and Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro [FAPERJ E-26/202.527/2019]. S.A.P. is the recipient of a productivity fellowship from Conselho Nacional de Pesquisa e Desenvolvimento Tecnológico (CNPq). RA-P was supported in part by Conselho Nacional de Desenvolvimento Científico e Tecnológico [CNPq 305487/2015-9]. This work was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES) - Finance Code 001. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
MLMR, RMZ-O, and RA-P helped in conceptualization; MLMR, RAC, FB-S, DG, MP, and MHGF-C contributed to methodology; MLMR, SAP, IDFG, RO-C, MHGF-C, and RA-P formally analyzed and investigated; MLMR and MHGF-C wrote the original draft preparation; all authors wrote the review and edited; SAP, RMZ-O, and RA-P helped in funding acquisition.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics Approval
The inclusion of human patients in this study was approved by the Research Ethics Committee of the Instituto Nacional de Infectologia Evandro Chagas/Fundação Oswaldo Cruz (INI/Fiocruz) (CAAE: 62420416.1.0000.5262) and HUPE/UERJ (CAAE: 62420416.1.3001.5259). The Ethics Committee for Animal Use of Fundação Oswaldo Cruz (LW-9/18) approved the study of dogs and cats.
Consent for Publication
All participants signed a consent permitting the use of their data in the publication.
Consent to Participate
All participants signed a consent to participate in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Handling editor: Vishnu Chaturvedi
Rights and permissions
About this article
Cite this article
Ramos, M.L.M., Coelho, R.A., Brito-Santos, F. et al. Comparative Analysis of Putative Virulence-Associated Factors of Microsporum canis Isolates from Human and Animal Patients. Mycopathologia 185, 665–673 (2020). https://doi.org/10.1007/s11046-020-00470-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11046-020-00470-9