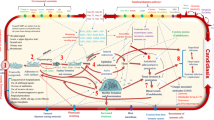
Abstract
Aspergillus terreus is an emerging opportunistic fungal pathogen that causes invasive aspergillosis in immunocompromised individuals. The main risk group of individuals for this organism is leukopenic patients, individuals having cancers, bone marrow transplant persons and those who have immunological disorders. The lack of early diagnostic marker for A. terreus and intrinsic resistance to Amphotericin B, further limits the successful therapy of A. terreus-associated infections. The germination of inhaled conidia is the key step to establish successful invasion in host tissues or organs. Thus, profiling of expressed proteins during germination of conidia not only shed light on proteins that are involved in invasion or virulence but may also provide early diagnostic markers. We used nanoLC-Q-TOF to study the proteome of germinating conidia (at 16 h time points) of A. terreus. We observed expression of 373 proteins in germinating conidia of A. terreus. A total of 74 proteins were uncharacterized in the database. The expressed proteins were associated with various processes like cell wall modulation, virulence factors and secondary metabolite biosynthesis. The most abundant proteins were associated with protein biosynthesis, carbohydrate metabolism and unknown functions. Among virulent proteins, mitogen-activated protein kinase (hog1) and mitogen-activated protein kinase (mpkC) are key virulent proteins observed in our study. We observed 7 enzymes from terretonin and 10 enzymes from geodin mycotoxin biosynthesis pathway. Interestingly, we observed expression of terrelysin protein, associated with blood cell lysis. Quantitative RT-PCR analysis showed 26-fold increase in transcripts encoding for dihydrogeodin oxidase and 885-fold for terrelysin gene in germinating conidia in comparison to conidia. Further, we propose that terrelysin protein and secondary metabolite such as geodin could be explored as diagnostic marker for A. terreus-associated infections.





Similar content being viewed by others
References
Fernandez MS, Rojas FD, Cattana ME, Sosa Mde L, Mangiaterra ML, Giusiano GE. Aspergillus terreus complex: an emergent opportunistic agent of onychomycosis. Mycoses. 2013;56(4):477–81. doi:10.1111/myc.12061.
Hachem R, Gomes MZ, El Helou G, El Zakhem A, Kassis C, Ramos E, Jiang Y, Chaftari AM, Raad II. Invasive aspergillosis caused by Aspergillus terreus: an emerging opportunistic infection with poor outcome independent of azole therapy. J Antimicrob Chemother. 2014;69(11):3148–55. doi:10.1093/jac/dku241.
Elsawy A, Faidah H, Ahmed A, Mostafa A, Mohamed F. Aspergillus terreus meningitis in immunocompetent patient: a case report. Front Microbiol. 2015;6:1353. doi:10.3389/fmicb.2015.01353.
Slesiona S, Gressler M, Mihlan M, Zaehle C, Schaller M, Barz D, Hube B, Jacobsen ID, Brock M. Persistence versus escape: Aspergillus terreus and Aspergillus fumigatus employ different strategies during interactions with macrophages. PLoS ONE. 2012;7(2):e31223. doi:10.1371/journal.pone.0031223.
Slesiona S, Ibrahim-Granet O, Olias P, Brock M, Jacobsen ID. Murine infection models for Aspergillus terreus pulmonary aspergillosis reveal long-term persistence of conidia and liver degeneration. J Infect Dis. 2012;205(8):1268–77. doi:10.1093/infdis/jis193.
Kathuria S, Sharma C, Singh PK, Agarwal P, Agarwal K, Hagen F, Meis JF, Chowdhary A. Molecular epidemiology and in vitro antifungal susceptibility of Aspergillus terreus species complex isolates in Delhi, India: evidence of genetic diversity by amplified fragment length polymorphism and microsatellite typing. PLoS ONE. 2015;10(3):e0118997. doi:10.1371/journal.pone.0118997.
Thakur R, Anand R, Tiwari S, Singh AP, Tiwary BN, Shankar J. Cytokines induce effector T-helper cells during invasive aspergillosis; what we have learned about T-helper cells? Front Microbiol. 2015;6:429. doi:10.3389/fmicb.2015.00429.
Croft CA, Culibrk L, Moore MM, Tebbutt SJ. Interactions of Aspergillus fumigatus conidia with airway epithelial cells: a critical review. Front Microbiol. 2016;7:472. doi:10.3389/fmicb.2016.00472.
Clemons KV, Shankar J, Stevens DA. Mycologic endocrinology. In: Lyte M, Freestone PPE, editors. Microbial endocrinology: interkingdom signaling in infectious disease and health. New York: Springer New York; 2010. p. 269–290. doi: 10.1007/978-1-4419-5576-0_15.
Arnaud MB, Cerqueira GC, Inglis DO, Skrzypek MS, Binkley J, Chibucos MC, Crabtree J, Howarth C, Orvis J, Shah P, Wymore F, Binkley G, Miyasato SR, Simison M, Sherlock G, Wortman JR. The aspergillus genome database (AspGD): recent developments in comprehensive multispecies curation, comparative genomics and community resources. Nucleic acids research 40 (Database issue):D653-659; 2012. doi: 10.1093/nar/gkr875.
Kniemeyer O. Proteomics of eukaryotic microorganisms: the medically and biotechnologically important fungal genus Aspergillus. Proteomics. 2011;. doi:10.1002/pmic.201100087.
Suh M-J, Fedorova ND, Cagas SE, Hastings S, Fleischmann RD, Peterson SN, Perlin DS, Nierman WC, Pieper R, Momany M. Development stage-specific proteomic profiling uncovers small, lineage specific proteins most abundant in the Aspergillus Fumigatus conidial proteome. Proteome Sci. 2012;10(1):30. doi:10.1186/1477-5956-10-30.
Gautam P, Shankar J, Madan T, Sirdeshmukh R, Sundaram CS, Gade WN, Basir SF, Sarma PU. Proteomic and transcriptomic analysis of Aspergillus fumigatus on exposure to amphotericin B. Antimicrob Agents Chemother. 2008;52(12):4220–7. doi:10.1128/aac.01431-07.
Kubitschek-Barreira PH, Curty N, Neves GWP, Gil C, Lopes-Bezerra LM. Differential proteomic analysis of Aspergillus fumigatus morphotypes reveals putative drug targets. J Proteomics. 2013;78:522–34. doi:10.1016/j.jprot.2012.10.022.
Tiwari S, Thakur R, Goel G, Shankar J. Nano-LC-Q-TOF analysis of proteome revealed germination of Aspergillus flavus conidia is accompanied by MAPK signalling and cell wall modulation. Mycopathologia. 2016;181(11):769–86. doi:10.1007/s11046-016-0056-x.
Kandhavelu J, Demonte NL, Namperumalsamy VP, Prajna L, Thangavel C, Jayapal JM, Kuppamuthu D. Data set of Aspergillus flavus induced alterations in tear proteome: understanding the pathogen-induced host response to fungal infection. Data Brief. 2016;9:888–94. doi:10.1016/j.dib.2016.11.003.
Saritha M, Singh S, Tiwari R, R Goel, Nain L. Do cultural conditions induce differential protein expression: profiling of extracellular proteome of Aspergillus terreus CM20. Microbiol Res. 2016;192:73–83. doi:10.1016/j.micres.2016.06.006.
Louis B, Waikhom SD, Roy P, Bhardwaj PK, Singh MW, Chandradev SK, Talukdar NC. Invasion of Solanum tuberosum L. by Aspergillus terreus: a microscopic and proteomics insight on pathogenicity. BMC Res Notes. 2014;7:350. doi:10.1186/1756-0500-7-350.
Champer J, Ito JI, Clemons KV, Stevens DA, Kalkum M. Proteomic analysis of pathogenic fungi reveals highly expressed conserved cell wall proteins. J Fungi (Basel, Switzerland) 2016. doi:10.3390/jof2010006.
Leng W, Liu T, Li R, Yang J, Wei C, Zhang W, Jin Q. Proteomic profile of dormant Trichophyton rubrum conidia. BMC Genom. 2008;9(1):303. doi:10.1186/1471-2164-9-303.
Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;. doi:10.1016/0003-2697(76)90527-3.
Garderes J, Bedoux G, Koutsouveli V, Crequer S, Desriac F, Pennec GL. Lipopolysaccharides from commensal and opportunistic bacteria: characterization and response of the immune system of the host sponge Suberites domuncula. Mar Drugs. 2015;13(8):4985–5006. doi:10.3390/md13084985.
Gotz S, Garcia-Gomez JM, Terol J, Williams TD, Nagaraj SH, Nueda MJ, Robles M, Talon M, Dopazo J, Conesa A. High-throughput functional annotation and data mining with the Blast2GO suite. Nucleic Acids Res. 2008;36(10):3420–35. doi:10.1093/nar/gkn176.
Szklarczyk D, Franceschini A, Wyder S, Forslund K, Heller D, Huerta-Cepas J, Simonovic M, Roth A, Santos A, Tsafou KP, Kuhn M, Bork P, Jensen LJ, von Mering C. STRING v10: protein-protein interaction networks, integrated over the tree of life. Nucleic Acids Research 43 (Database issue):D447-452. 2015. doi:10.1093/nar/gku1003.
Ye J, Coulouris G, Zaretskaya I, Cutcutache I, Rozen S, Madden TL. Primer-BLAST: a tool to design target-specific primers for polymerase chain reaction. BMC Bioinform. 2012;13:134. doi:10.1186/1471-2105-13-134.
Monteiro JP, Clemons KV, Mirels LF, Coller JA Jr, Wu TD, Shankar J, Lopes CR, Stevens DA. Genomic DNA microarray comparison of gene expression patterns in Paracoccidioides brasiliensis mycelia and yeasts in vitro. Microbiology. 2009;155(Pt 8):2795–808. doi:10.1099/mic.0.027441-0.
Lass-Florl C, Grif K, Kontoyiannis DP. Molecular typing of Aspergillus terreus isolates collected in Houston, Texas, and Innsbruck, Austria: evidence of great genetic diversity. J Clin Microbiol. 2007;45(8):2686–90. doi:10.1128/jcm.00917-07.
Blum G, Perkhofer S, Grif K, Mayr A, Kropshofer G, Nachbaur D, Kafka-Ritsch R, Dierich MP, Lass-Florl C. A 1-year Aspergillus terreus surveillance study at the University Hospital of Innsbruck: molecular typing of environmental and clinical isolates. Clin Microbiol Infect. 2008;14(12):1146–51. doi:10.1111/j.1469-0691.2008.02099.x.
Chowdhary A, Sharma C, Kathuria S, Hagen F, Meis JF. Prevalence and mechanism of triazole resistance in Aspergillus fumigatus in a referral chest hospital in Delhi, India and an update of the situation in Asia. Front Microbiol. 2015;. doi:10.3389/fmicb.2015.00428.
Badiee P, Kordbacheh P, Alborzi A, Malekhoseini S, Ramzi M, Mirhendi H, Mahmoodi M, Shakiba E. Study on invasive fungal infections in immunocompromised patients to present a suitable early diagnostic procedure. Int J Infect Dis. 2009;13(1):97–102. doi:10.1016/j.ijid.2008.04.011.
Lamoth F. Galactomannan and 1, 3-β-d-glucan testing for the diagnosis of invasive aspergillosis. J Fungi. 2016;2(3):22.
Barton RC. Laboratory diagnosis of invasive aspergillosis: from diagnosis to prediction of outcome. Scientifica. 2013;2013:29. doi:10.1155/2013/459405.
Lass-Flörl C, Alastruey-Izquierdo A, Cuenca-Estrella M, Perkhofer S, Rodriguez-Tudela JL. In vitro activities of various antifungal drugs against Aspergillus terreus: global assessment using the methodology of the european committee on antimicrobial susceptibility testing. Antimicrob Agents Chemother. 2009;53(2):794–5. doi:10.1128/AAC.00335-08.
Sutton DA, Sanche SE, Revankar SG, Fothergill AW, Rinaldi MG. In vitro amphotericin B resistance in clinical isolates of Aspergillus terreus, with a head-to-head comparison to voriconazole. J Clin Microbiol. 1999;37(7):2343–5.
Bhabhra R, Miley MD, Mylonakis E, Boettner D, Fortwendel J, Panepinto JC, Postow M, Rhodes JC, Askew DS. Disruption of the Aspergillus fumigatus gene encoding nucleolar protein CgrA impairs thermotolerant growth and reduces virulence. Infect Immun. 2004;72(8):4731–40. doi:10.1128/iai.72.8.4731-4740.2004.
Renshaw H, Vargas-Muniz JM, Richards AD, Asfaw YG, Juvvadi PR, Steinbach WJ. Distinct roles of myosins in Aspergillus fumigatus hyphal growth and pathogenesis. Infect Immun. 2016;84(5):1556–64. doi:10.1128/iai.01190-15.
Bruder Nascimento AC, Dos Reis TF, de Castro PA, Hori JI, Bom VL, de Assis LJ, Ramalho LN, Rocha MC, Malavazi I, Brown NA, Valiante V, Brakhage AA, Hagiwara D, Goldman GH. Mitogen activated protein kinases SakA(HOG1) and MpkC collaborate for Aspergillus fumigatus virulence. Mol Microbiol. 2016;100(5):841–59. doi:10.1111/mmi.13354.
McGoldrick CA, Gruver C, May GS. myoA of Aspergillus nidulans encodes an essential myosin I required for secretion and polarized growth. J Cell Biol. 1995;128(4):577–87.
Hohl TM, Feldmesser M. Aspergillus fumigatus: principles of pathogenesis and host defense. Eukaryot Cell. 2007;6(11):1953–63. doi:10.1128/ec.00274-07.
Lamarre C, Sokol S, Debeaupuis JP, Henry C, Lacroix C, Glaser P, Coppee JY, Francois JM, Latge JP. Transcriptomic analysis of the exit from dormancy of Aspergillus fumigatus conidia. BMC Genom. 2008;. doi:10.1186/1471-2164-9-417.
Cagas SE, Jain MR, Li H, Perlin DS. The proteomic signature of Asergillus fumigatus during early development. Mol Cell Proteomics. 2011;. doi:10.1074/mcp.M111.010108.
Ozdemir HG, Kandemir H, Curuk A, Ilkit M, Seyedmousavi S. Infrequent production of xanthomegnin by fungal strains recovered from patients with ocular mycoses. Mycopathologia. 2016;181(3–4):241–6. doi:10.1007/s11046-015-9970-6.
Lewis RE, Wiederhold NP, Chi J, Han XY, Komanduri KV, Kontoyiannis DP, Prince RA. Detection of gliotoxin in experimental and human aspergillosis. Infect Immun. 2005;73(1):635–7. doi:10.1128/IAI.73.1.635-637.2005.
Koo S, Thomas HR, Daniels SD, Lynch RC, Fortier SM, Shea MM, Rearden P, Comolli JC, Baden LR, Marty FM. A breath fungal secondary metabolite signature to diagnose invasive aspergillosis. Clin Infect Dis. 2014;59(12):1733–40. doi:10.1093/cid/ciu725.
Spikes S, Xu R, Nguyen CK, Chamilos G, Kontoyiannis DP, Jacobson RH, Ejzykowicz DE, Chiang LY, Filler SG, May GS. Gliotoxin production in Aspergillus fumigatus contributes to host-specific differences in virulence. J Infect Dis. 2008;197(3):479–86. doi:10.1086/525044.
Scharf DH, Heinekamp T, Remme N, Hortschansky P, Brakhage AA, Hertweck C. Biosynthesis and function of gliotoxin in Aspergillus fumigatus. Appl Microbiol Biotechnol. 2012;93(2):467–72. doi:10.1007/s00253-011-3689-1.
Shankar J. An overview of toxins in Asergillus associated with pathogenesis. Int J LifeSc Bt Pharm Res. 2013;2(2):16–31.
Cowen LE. The evolution of fungal drug resistance: modulating the trajectory from genotype to phenotype. Nat Rev Microbiol. 2008;6(3):187–98. doi:10.1038/nrmicro1835.
Alcazar-Fuoli L, Mellado E. Ergosterol biosynthesis in Aspergillus fumigatus: its relevance as an antifungal target and role in antifungal drug resistance. Front Microbiol. 2012;3:439. doi:10.3389/fmicb.2012.00439.
Nayak AP, Blachere FM, Hettick JM, Lukomski S, Schmechel D, Beezhold DH. Characterization of recombinant terrelysin, a hemolysin of Aspergillus terreus. Mycopathologia. 2011;171(1):23–34. doi:10.1007/s11046-010-9343-0.
Nayak AP, Green BJ, Beezhold DH. Fungal hemolysins. Med Mycol. 2013;51(1):1–16. doi:10.3109/13693786.2012.698025.
Chamilos G, Kontoyiannis DP. Defining the diagnosis of invasive aspergillosis. Med Mycol. 2006;44(Supplement_1):S163–73. doi:10.1080/13693780600823258.
Acknowledgements
We are thankful to Department of Biotechnology and Bioinformatics, Jaypee University of Information Technology, for providing facilities to carry out the work and Ph.D. fellowship.
Author information
Authors and Affiliations
Contributions
RT and JS designed the project; RT performed the work; RT and JS analyzed the data and wrote the manuscript; both authors reviewed and approved manuscript; JS contributed reagents/materials/analysis tools.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that no financial and competing interest exists.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Supplementary file 1
Total number of proteins expressed in germinating conidia of Aspergillus terreus (XLSX 37 kb)
Supplementary file 2
Identification of proteins from Aspergillus terreus in germinating conidia and their general function annotated from UniProt database (DOCX 21 kb)
Supplementary file 3
Number of proteins expressed in Aspergillus terreus conidia (XLSX 13 kb)
Supplementary file 4
Conidial proteins of Aspergillus terreus and their function annotated from UniProt database (DOCX 14 kb)
Supplementary file 5
Identification of proteins of Aspergillus terreus germinating conidia involved in protein biosynthesis and amino acid metabolism (DOCX 16 kb)
Supplementary file 6
Other secondary metabolite pathway proteins identified during germination of Aspergillus terreus conidia (DOCX 13 kb)
Supplementary file 7
List of proteins used for interacting network analysis using String database (DOCX 15 kb)
Supplementary table-1
Primers used for quantitative expression analysis (DOCX 12 kb)
Supplementary table-2
Comparative analysis of expressed proteins of A. terreus germinating conidia with proteomic studies of A. fumigatus and A. flavus germinating conidia (DOCX 16 kb)
Supplementary figure-1
Figure represents the SDS-PAGE separation of A. terreus proteins extracted from conidia and germinating conidia (PDF 36 kb)
Supplementary figure-2
Protein–protein interaction network of identified proteins of A. terreus germinating conidia (TIFF 4436 kb)
Rights and permissions
About this article
Cite this article
Thakur, R., Shankar, J. Proteome Profile of Aspergillus terreus Conidia at Germinating Stage: Identification of Probable Virulent Factors and Enzymes from Mycotoxin Pathways. Mycopathologia 182, 771–784 (2017). https://doi.org/10.1007/s11046-017-0161-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11046-017-0161-5