Abstract
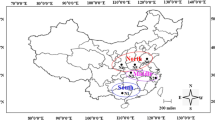
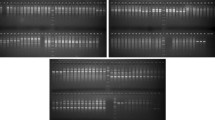
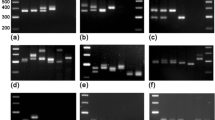
Morphological features and Inter Simple Sequence Repeat (ISSR) polymorphism were employed to analyse 21 Corynespora cassiicola isolates obtained from a number of Hevea clones grown in rubber plantations in Malaysia. The C. cassiicola isolates used in this study were collected from several states in Malaysia from 1998 to 2005. The morphology of the isolates was characteristic of that previously described for C. cassiicola. Variations in colony and conidial morphology were observed not only among isolates but also within a single isolate with no inclination to either clonal or geographical origin of the isolates. ISSR analysis delineated the isolates into two distinct clusters. The dendrogram created from UPGMA analysis based on Nei and Li’s coefficient (calculated from the binary matrix data of 106 amplified DNA bands generated from 8 ISSR primers) showed that cluster 1 encompasses 12 isolates from the states of Johor and Selangor (this cluster was further split into 2 sub clusters (1A, 1B), sub cluster 1B consists of a unique isolate, CKT05D); while cluster 2 comprises of 9 isolates that were obtained from the other states. Detached leaf assay performed on selected Hevea clones showed that the pathogenicity of representative isolates from cluster 1 (with the exception of CKT05D) resembled that of race 1; and isolates in cluster 2 showed pathogenicity similar to race 2 of the fungus that was previously identified in Malaysia. The isolate CKT05D from sub cluster 1B showed pathogenicity dissimilar to either race 1 or race 2.







Similar content being viewed by others
Abbreviations
- PDI:
-
Percent disease intensity
- CMI:
-
Commonwealth Mycology Institute
- UBC:
-
University of British Colombia
References
Farr DF, Rossman AY, Palm ME, McCray EB. Fungal databases. Systematic Botany & Mycology Laboratory, ARS, USDA. Retrieved Dec 13, 2007, from http://nt.ars-grin.gov/fungaldatabases/.
Deighton FC. Preliminary list of fungi and diseases of plants in Sierra Leone. Kew Bull. 1936;7:397–424.
Ramakrishnan TS, Pillay R. Leaf spot of rubber caused by Corynespora cassiicola (Berk. & Curt.). Wei Rubber Bd Bull. 1961;5:32–5.
Newsam S. Pathology division report. Rubber Res Inst Malaysia 1961;63–70.
Awoderu VA. New leaf spot of para rubber (Hevea brasiliensis) in Nigeria. Plant Dis Reptr. 1969;53(5):406–8.
Teoh CH. Corynespora leaf fall of Hevea in West Java. Malaysian Plant Protection Society. Newsletter. 1983;7(2):12–3.
Junqeuira NTV, Gasparotto L, Moraes VHF, Silva HM, Lim TM. New diseases caused by virus, fungi and also bacterium on rubber from Brazil and their impact on international quarantine. In: Proceeding of the regional conference on plant quarantine support for agricultural development, Kuala Lumpur, Malaysia, 10–12 December 1985. p. 253–60.
Liyanage AS, Jayasinghe CK, Liyanage NIS, Jayaratne AHR. Corynespora leaf spot disease of rubber (Hevea brasiliensis)––A new report. J Rubber Res Inst Sri Lanka. 1986;65:47–50.
Pongthep K. Corynespora disease of Hevea in Thailand. IRRDB’s symposium on pathology of Hevea in Chieng Mai, Thailand, 2–3rd Nov. 1987.
Rahman MA. Diseases of Hevea brasiliensis in Bangladesh. Bano Biggyyan Patrika. 1988;17:73–9.
Dung PT, Hoan NT. Corynespora leaf fall on rubber in Vietnam, a New record. In: Proceeding of IRRDB Symposium 1999. Hainan Publishing House. p. 273–5.
Jinji P, Zhang X, Qi Y, Xie Y, Zhang H, Zhang H. First record of Corynespora leaf fall disease of Hevea rubber tree in China. Aust Plant Dis Notes. 2007;2:35–6. doi:10.1071/DN07017.
Tan AM, Lo TP, Vadivel G, Bachik MS, Yoon KP. Survey of major leaf diseases of rubber in Peninsular Malaysia. RRIM Planters’ Bull. 1992;211:51–62.
Jayashinghe CK, Silva WPK. Current status of Corynespora leaf fall in Sri Lanka. In: Proceeding workshop on Corynespora leaf fall disease of Hevea rubber, 16–17 December 1996, Medan, Indonesia. p. 15–9.
Sinulingga W, Suwarto, Soepena H. Current status of Corynespora leaf fall in Indonesia. In: Proceeding workshop on Corynespora leaf fall disease of Hevea rubber, 16–17 December 1996, Medan, Indonesia. p. 29–36.
Breton F, D’Auzac J, Garcia D, Sanier C, Eschbach JM. Recent research on Corynespora cassiicola/Hevea brasiliensis interaction. In: Proceeding workshop on Corynespora leaf fall disease of Hevea rubber, 16–17 December 1996, Medan, Indonesia. p. 49–78.
Shamsul Kamar AS, Shamsuri MH. Current status of Corynespora leaf fall in Malaysia. In: Proceeding workshop on Corynespora leaf fall disease of Hevea rubber, 16–17 December 1996, Medan, Indonesia. p. 21–8.
Ismail H, Jeyanayagi I. Occurrence and identification of physiological races of Corynespora cassiicola of Hevea. In: Proceeding of IRRDB Symposium 1999. Hainan Publishing House. p. 263–72.
Darmono TW, Darussamin A, Pawirosoemardjo S. Variation among isolates of Corynespora cassiicola associated with Hevea brasiliensis in Indonesia. In: Proceeding workshop on Corynespora leaf fall disease of Hevea rubber, 16–17 December 1996, Medan, Indonesia. p. 79–91.
Silva WPK, Karunanayake EH, Wijesundera RLC, Priyanka UMS. Genetic variation in Corynespora cassiicola: a possible relationship between host origin and virulence. Mycol Res. 2003;107(5):567–71. doi:10.1017/S0953756203007755.
Saha T, Arun Kumar A, Sreena S, Joseph A, Kuruvilla Jacob C, Kothandaraman R, Nazeer MA. Genetic variability of Corynespora cassiicola infecting Hevea brasiliensis isolated from the traditional rubber growing areas in India. Indian J Nat Rubber Res. 2000;13(1&2):1–10.
Atan S, Hamid NH. Differentiating races of Corynespora cassiicola using RAPD and internal transcribed spacer markers. J Rubber Res. 2003;6(1):58–64.
Romruensukharom P, Tragoonrung S, Vanavichit A, Toojinda T. Genetic variability of Corynespora cassiicola population in Thailand. J Rubber Res. 2005;8(1):38–49.
Othman R, Benong M, Ong SH, Ismail H. Strategies and development of resistant Hevea clones against Corynespora leaf fall. In: Proceeding workshop on Corynespora leaf fall disease of Hevea rubber, 16–17 December 1996, Medan, Indonesia. p. 177–94.
RRIM Malaysian Country Report, IRRDB Workshop on Corynespora Leaf Fall of Rubber, 6–9 June 2000, Malaysia and Indonesia 2000.
Doyle JJ, Doyle JL. Isolation of plant DNA from fresh tissue. Focus. 1990;12:13–5.
Philip S, Roy B. Molecular characterization of Corynespora cassiicola. In: A laboratory manual for international training on strategies for management of Corynespora leaf fall disease of H. brasiliensis. 18–29 April 2006, Rubber Research Institute of India. p. 8–16.
Mishra PK, Tewari JP, Clear RM, Turkington TK. Molecular genetic variation and geographical structuring in Fusarium graminearum. Ann Appl Biol. 2004;145:299–307. doi:10.1111/j.1744-7348.2004.tb00387.x.
Nei M, Li WH. Mathematical model for studying genetic variation in terms of restriction endonucleases. Proc Natl Acad Sci USA. 1979;76:5269–73. doi:10.1073/pnas.76.10.5269.
Pavlíček A, Hrdá Š, Flegr J. FreeTree––Treeware program for construction of phylogenetic trees on the basis of distance data and for bootstrap/jackknife analysis of the trees robustness. Application in the RAPD analysis of genus Frenkelia. Folia Biologica (Praha). 1999;45:97–9.
Page RDM. TREEVIEW: an application to display phylogenetic trees on personal computers. Comput Appl Biosci. 1996;12:357–8.
Liyanage AS, Jayasinghe CK, Liyanage NIS, Jayaratne AHR. Corynespora leaf spot disease of rubber (Hevea brasiliensis)––A new report. J Rubber Res Inst Sri Lanka. 1986;65:47–50.
Chee KH. Studies on sporulation, pathogenicity and epidemiology of Corynespora. J Nat Rubber Res. 1988;1(3):21–9.
Ellis MB, Holliday P. Corynespora cassiicola. C. M. I. description of pathogenic fungi and bacteria. No. 303. 1971.
Mushrif SK. Morphology, physiology and survival of Corynespora cassiicola (Bert.&Curt.) Wei. In: K Jacob, editor. Corynespora leaf disease of Hevea brasiliensis. Strategy for management. Rubber Research Institute of India; 2006. p. 26–32.
Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215(3):403–10.
Spencer JA, Walter HJ. Variation in certain isolates of Corynespora cassiicola. Phytopathology. 1969;59:58–60.
Onesirosan PT, Arny DC, Durbin RD. Host specificity of Nigerian and North American isolates of Corynespora cassiicola. Phytopathology. 1974;64:1364–7.
Duarte MLR, Asano S, Albuquerque FC. Comparative study of the morphological and physical characteristics of two Corynespora cassiicola isolates. Fitopatologia Brasileira. 1983;8:205–14.
Dung Phan Thanh. Studies on Corynespora cassiicola (Bert. & Curt.) Wei on Rubber. Master Thesis, Universiti Pertanian Malaysia (UPM) 1995.
Silva WPK, Deverall BJ, Lyon BR. Molecular, physiological and pathological characterization of Corynespora leaf spot fungi from rubber plantations in Sri Lanka. Plant Pathol. 1998;47(3):267–77. doi:10.1046/j.1365-3059.1998.00245.x.
Gil-Lamaignere C, Roilides E, Hacker J, Muller FMC. Molecular typing for fungi––a critical review of the possibilities and limitation of currently and future methods. Clin Microbiol Infect 2003;9:172–85. doi:10.1046/j.1469-0691.2003.00649.x.
International Atomic Energy Agency. Mutant germplasm characterization using molecular markers: a Manual, IAEA-TCS-19, IAEA. 2002; Introduction 11.
Zietkiewicz E, Rafalski A, Labuda D. Genome fingerprinting by simple sequence repeat (SSR)-anchored polymerase chain reaction amplification. Genomics. 1994;20:176–83. doi:10.1006/geno.1994.1151.
Goldwin ID, Aitken AB, Smith LW. Application of Inter simple sequence repeats (ISSR) markers to plant genetics. Electrophoresis. 1997;18:1524–8. doi:10.1002/elps.1150180906.
Burgess T, Wingfield MJ, Wingfield BW. Simple sequence repeat markers distinguish among morphotypes of Sphaeropsis sapinea. Appl Environ Microbiol. 2001;67(1):354–62. doi:10.1128/AEM.67.1.354-362.2001.
Meng X, Chen W. Applications of AFLP and ISSR techniques in detecting genetic diversity in the soybean brown stem rot pathogen Phialophora gregata. Mycol Res. 2001;105(8):936–40. doi:10.1016/S0953-7562(08)61949-8.
Van Der Merwe NA, Wingfield BD, Wingfield MJ. Primers for the amplification of sequence-characterized loci in Cryphonectria cubensis populations. Mol Ecol Notes. 2003;3:494–7. doi:10.1046/j.1471-8286.2003.00508.x.
Kauserud H, Schumacher T. Regional and local population structure of the pioneer wood-decay fungus Trichaptum abietinum. Mycologia. 2003;95(3):416–25.
Menzies JG, Bakkeren G, Matheson F, Procunier JD, Woods S. Use of inter-simple sequence repeats and amplified fragment length polymorphisms to analyze genetic relationships among small grain-infecting species of Ustilago. Phytopathology. 2003;93:167–75. doi:10.1094/PHYTO.2003.93.2.167.
Kauserud H. Widespread vegetative compatibility groups in the dry-rot fungus Serpula lacrymans. Mycologia. 2003;96(2):232–9. doi:10.2307/3762059.
Elena Estrada M, Camacho MV, Benito C. The molecular diversity of different isolates of Beauveria Bassiana (Bals.) Vuill. As assessed using intermicrosatellites (ISSRs). Cell Mol Biol Lett. 2007;12:240–52. doi:10.2478/s11658-006-0069-4.
Acknowledgements
The study presented in this paper was undertaken as part of a doctoral study project which was supported by Universiti Putra Malaysia (UPM) and Malaysian Rubber Board (MRB). Nguyen Anh Nghia, a research officer from Rubber Research Institute of Vietnam (RRIV), was financially sponsored by a Scholarship from the Ministry of Education, Vietnam Government. The authors would like to thank Dr. Shamsul Bahari from the MRB for granting permission to use the facilities for conidial morphology study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nghia, N.A., Kadir, J., Sunderasan, E. et al. Morphological and Inter Simple Sequence Repeat (ISSR) Markers Analyses of Corynespora cassiicola Isolates from Rubber Plantations in Malaysia. Mycopathologia 166, 189–201 (2008). https://doi.org/10.1007/s11046-008-9138-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11046-008-9138-8