Abstract
The Internet of Bio-Nano Things concept (IoBNT) emerged from the need to establish connections between biological nanomachines, the intra-body nanonetwork, and the cyber internet to facilitate information exchange. While extensive research has concentrated on optimizing communication efficiency among nanodevices within networks, challenges such as IoBNT security and the interface linking nanonetwork to the internet have remained unaddressed. Consequently, this study introduces a privacy scheme designed to operate atop the Physical Cyber Interface (pHCI) within the IoBNT framework. Our proposed chaotic system derives its foundation from the command signals issued by medical personnel to pHCI devices implanted within the human body. It employs a concealed version of features generated through a Modified Quadratic Map (MQM) to enhance the privacy of patient information and to ensure a precise dosage release. Additionally, our scheme incorporates Binary Phase Shifting Key (BPSK) modulation through the incorporation of a carrier wave, along with feature extraction with zero-crossing rates. This privacy scheme significantly amplifies the key space, thereby guaranteeing an accurate right dose release with the protection of patient privacy. To assess the performance of our proposed scheme, we evaluate its operation on top of the pHCI device using various performance metrics. Subsequently, we study its performance by employing multi-compartmental models in both the forward and reverse pHCI directions of the IoBNT paradigm. The results from our simulation model clearly illustrate that the IoBNT-based privacy scheme has potential to enhance the delivery of therapeutic drugs to target cells while effectively addressing privacy concerns. An evaluation of performance metrics for two binary codes (thermal and light) reveals sensitivity and specificity rates of 95.333% and 95%, 100%, and 100%, respectively. Furthermore, the performance of our proposed privacy scheme, as measured by EER, accuracy, NPV, and PPV, has proven to be highly satisfactory. Hence, our proposed scheme makes significant role in enhancing the security of the physical cyber interface device while remaining cost-effective, and ensuring the safety of patients' life and confidentiality.
Similar content being viewed by others
1 Introduction
Nanotechnology [1] has introduced novel concepts, methods, and devices with the potential to enhance existing technologies and lead in entirely new scientific breakthroughs. Bio-nanotechnology, founded on the manipulation of materials at the molecular and nanoparticle levels, offers small biocompatible devices (artificial machines) capable of manipulating bio-materials at the system and organism levels. These artificial machines perform various functions, including sensing, capturing, storing, releasing, synthesizing biological molecules, acquiring and expending energy, mobility, actuation, replication, and termination, as described in [2]. Doxorubicin (Dox) is a commonly used anti-cancer therapy effective against a wide range of tumors. However, Dox use is limited by the risk of cardiotoxicity, which imposes constraints on dosing duration. Consequently, researchers are actively seeking optimal dosing regimens that maximize anti-tumor efficacy while mitigating the cardiac toxicity associated with plasma concentration [3]. As a result, nanotechnology can provide drug carriers in the form of nanoparticles (NPs) designed for delivery and release at the tumor site. Furthermore, nanotechnology also suggests that Molecular Communication (MC) can be employed to connect such artificial machines. The significance of MC lies in its ability to deeply understand complex diseases, such as cancer, by identifying fundamental intracellular and extracellular processes within the context of interactive artificial machines and managing them comprehensively through an externally controlled system. The IoBNT [4,5,6,7], which is based on encrypted pHCI, represents a promising system for ensuring privacy and establishing connections between the MC environment and the external environment, including the Internet.
Consequently, MC has potential applications in nanomedicine, an area where the subject of Targeted Drug Delivery Systems (TDDS) is currently under extensive investigation [8,9,10,11]. The primary objective of TDDS is to deliver specific medicines precisely where they are needed (the targeted site) while preventing the drugs from adversely affecting other healthy parts of the body [12]. A TDDS methodology based on MC involves the use of artificial nano-devices as transmitters to transport drug particles, with drug molecules serving as carriers of information. Additionally, the blood plasma network functions as a communication channel, with the targeted or tumor tissue acting as the receiving nano-machine [8, 9].
Designing and implementing a functional IoBNT presents several challenges. These challenges include the construction and advancement of artificial nano-devices, the molecular communication structure within the nano-network, and establishment of an interface between nanonetwork and Internet. Further, previous studies involving IoBNT have primarily focused on the effectiveness of communication among artificial nanomachines within a specific nano-network, without addressing the need to secure the signals transmitted through the network. Furthermore, these signals play a crucial role in healthcare delivery, particularly in the separation of drug particles within the vascular channel until they reach their targeted location. These studies, however, tend to overlook the potential adverse effects on healthy cells. Moreover, the IoBNT system can result in the extended persistence of medicine in the vascular system. Additionally, certain disorders, such as rapidly growing and highly localized cancers, require extremely precise treatment. Consequently, inadequate localization of tumor cells (the targeted site) can have negative repercussions. Moreover, there is a need for research on rate-control techniques to manage the release of information molecules based on environmental conditions [13, 14]. Furthermore, challenges related to storage speed, storage capacity, and the management of these processes in hybrid devices remain unresolved. The tracking and localization of artificial nano-devices, as well as network security, are also areas that necessitate in-depth study. Each of these topics remains unresolved in the field of nano-communication studies.
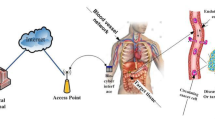
As mentioned earlier, IoBNT still suffers from a security issue. Thus, privacy measures have become essential to safeguard patient life, ensure accurate dosages, and prevent external attacks on the IoBNT paradigm. Employing techniques like the MQM, which has been suggested in the literature and is utilized in the current study, is one approach for addressing these risks. Furthermore, this paper focuses on IoBNT and its medical applications in healthcare surveillance systems and drug delivery, as depicted in Fig. 1. In the present study, we introduce an IoBNT cipher model using two distinct approaches. Firstly, we introduce a cipher model based on a multi-compartmental model that utilizes a ciphered pHCI device, which operates based on command signals sent by medical personnel to the pHCI. This ensures the precise release of the correct drug within the targeted nano-network, thereby safeguarding patient life by delivering the proper dose without risk. Secondly, our study explores targeted nano-networks within the targeted tissue and investigates how to deliver therapeutic medications to certain cells (e.g., tumor cells) while minimizing side effects.
Our primary current contributions are as follows:
-
1.
Introducing a privacy system based on the command signals transmitted from medical personnel to the biocyber interface device.
-
2.
Developing a comprehensive end-to-end system for delivering therapeutic medications to target or diseased cells, encompassing the blood vessel channel, extracellular channel, and intracellular channel.
-
3.
Performance evaluation of the proposed model.
The paper is structured as follows: Section 2 describes the related work, and Section 3 presents the proposed system framework. Section 4 illustrates the proposed privacy scheme while Section 5 outlines the proposed multi-compartmental framework, covering both the forward and reverse directions for broadcasting a molecular signal from the blood plasma network to the tumor network. Section 6 introduces the simulations and numerical results of the proposed system. Finally, Section 7 concludes the paper and summarizing the findings and main conclusions.
2 Related work
Information Communication Technology (ICT) and the IoBNT are integrated to develop new drug delivery systems for drug targeting and providing a TDDS. The model presented in [15] introduces a descriptive scenario and IoBNT system used in an advanced healthcare delivery system. This model was employed to address one of IoBNT's primary challenges by illustrating the architecture and model of a bio-cyber interface that connects a biochemical signaling-based nanonetwork to a conventional electromagnetic-based network. In contrast, the Internet does not guarantee that the drug reaches the target cell in the model presented in [16]. Consequently, new values were introduced to ensure effective drug delivery to the targeted cell. This analysis concentrates on the system, including a bio-cyber interface and a blood vessel information propagation network leading to the location of the intra-body nanonetwork. In [12], the authors proposed a MC-based TDDS to deliver drug therapy to multiple sites, aiming to enhance TDDS effectiveness at diseased sites. In [17], a new TDDS paradigm for the development of DOX drug delivery systems are described, aiming to improve the therapeutic window (efficacy and safety) and address limitations of existing FDA-approved Dox formulations. In [3], a mathematical model for the cellular uptake and cytotoxicity of the anti-cancer drug DOX is presented. This model assumes sigmoidal, Hill-type dependence on drug-induced damage for cell survival. The results demonstrate that DOX cytotoxicity involves distinct intracellular and extracellular mechanisms. Drug-induced damage is expressed as the sum of two terms representing the peak values of intracellular and extracellular drug concentrations over time.
In contrast to earlier related research, which focused on delivering therapeutic drug molecules to infected cells at specific sites through conventional compartmental models, prior studies didn't incorporate the IoBNT model for active control and drug delivery to targeted sites, including the elimination process. Additionally, critical challenges such like IoBNT security and establishing a connection interface between the nanonetwork and the Internet have remained unaddressed. In response to these existing gaps, the proposed framework aims to introduce a privacy scheme that operates atop the pHCI within the IoBNT paradigm. This scheme is established to precisely regulate the concentration of drug molecules within the intended site while minimizing potential negative effects on neighboring healthy cells. The developed model reveals that the proposed IoBNT-based privacy scheme has a substantial promise for significantly improving the delivery of therapeutic drugs to target cells, while simultaneously enhancing privacy protections and addressing security concerns.
3 Proposed framework
The framework of proposed system is depicted in Fig. 1. The system framework, as shown in Fig. 1, encompasses six constituent units: Internet, access point, wireless channel, Ciphered pHCI device, blood vessel channel, and targeted tissue. These units, modeled as time-varying impulse responses, draw inspiration from conventional communication systems and operate as follows: Firstly, medical personnel transmit ciphered unique binary code orders over the Internet, which are designed to execute various tasks such as releasing drug particles, synthesizing compounds, and sensing suitable drug particles. The traditional Internet of Things (IoTs) is represented as \({{\varvec{x}}}_{1}({\varvec{t}})\) and \({{\varvec{x}}}_{2}({\varvec{t}})\). To secure the binary code sent by medical staff until it reaches the ciphered pHCI, we propose employing a ciphering technique. In the current paper, our focus is on modeling two phases: the first phase extends from the wireless channel to the blood vessel channel via the ciphered pH-cyber interface device, and the second phase spans from the bio-nanosensor to the medical personnel through the blood vessel. In the latter phase, the biochemical signal detected in the blood network by the bio-nanosensor is converted into an electrical signal. The bio-nanosensor detects information molecules in blood vessels generated by the bioluminescence reaction. Consequently, a ciphered pHCI conveys this signal to medical personnel over the Internet. The design of this model necessitates the achievement of two primary objectives. The first objective is to secure the coding transmitted by medical personnel via the Internet. The second objective is to realize the desired effects of therapeutic drugs, including the localization of drugs within targeted or specific cells, accomplished through an end-to-end system. Furthermore, another objective is to address the challenges of rapid absorption, dosage clearance, and the mitigation of side effects in other healthy areas of the body.
It is essential to explain how this system operates, from the medical staff on down to the drug delivery to the targeted cells (tumor cell). The medical staff transmits via the access point the Ciphered command information. As a result, the communication between \({{\varvec{x}}}_{1}\) (t), \({{\varvec{x}}}_{2}\) (t) and \({{\varvec{x}}}_{3}\) (t) are as the following:
where \({{\varvec{y}}}^{\left({\varvec{n}}\right)}\) is the input signal and * is the operator of convolution. The superscript n is the type of communication direction, where r is the reverse communication direction (from the targeted tissue to the medical personnel) and f is the forward communication direction (from the medical personnel to the targeted tissue).
Figure 1 illustrates an example of the IoBNT functional modules that inspired by previous work [18], which will be developed in the present proposed system model. We consider a healthcare delivery system scenario in which the DOX drug is delivered to the targeted cell (malignant cell) within the target tissue. In this scenario, the targeted nano-network consists of a group of therapeutic artificial machines (n \({\varvec{R}}{{\varvec{T}}}_{1}\), n \({{\varvec{R}}{\varvec{T}}}_{2}\), n \({\varvec{R}}{{\varvec{T}}}_{3}\) and bio-nano-sensor) have injected into the intra-body area network. A smart injection pump regulating the injection of DOX drug-loaded artificial machines or drug molecules is described in [19] while the transmission and propagation of DOX drugs can be accomplished by MC system found in [9, 10]. On the other hand, the bio-nano sensor senses around the targeted nano-network to detect any chemical signal. Inspired by the biological cell structure, the therapeutic artificial machines have equipped by receptors for binding with the specific ligand (drug), thereby the reception process is performed according to the ligand-receptor binding mechanism. Additionally, therapeutic artificial machines are capable of transmitting and receiving data (i.e., transceiver artificial nano-devices). It is assumed that n \({\varvec{R}}{{\varvec{T}}}_{1}\) is the transceiver artificial machine (similar to PEG ylated liposome) that encapsulates the desired drug, such as DOX, and it has the ability to receive stimuli signal and thus emitting Dox drug. The n \({{\varvec{R}}{\varvec{T}}}_{2}\) and n \({{\varvec{R}}{\varvec{T}}}_{3}\) are artificial machines that can also synthesize and release targeted molecules to aid in completion of the drug delivery system scenario.
In the present study, it is considered that drug molecules pass via multi channels to infected cells in targeted location. The first channel is an outside intra-body nano-network that connects medical personnel to a pHCI based on privacy signals. In order to make a liposome release of its contents inside the targeted location, a bio-nanosensor is instructed via a ciphered pHCI. Other channels are located inside the intra-body nano-network, while the propagation of the drug particles in these channels are executed by using the multi compartmental model.
4 The privacy model
4.1 Outside the intra-body Nano-network
The framework in the forward direction has two parts, as depicted in Fig. 1. The first stage transpires outside the intra-body nanonetwork, where the signal transmitted from the access point to the ciphered pHCI undergoes transformation into a binary code. This binary code is subsequently employed in logic gates to determine which stimuli affect the nano-carriers. In the current study, in accordance with the findings detailed in [15, 16], two stimuli, namely thermal and light, exert influence over the content of the liposomes. Each of these stimuli is represented by N binary code samples, and convolution is performed with a specialized mask for each stimulus code. Subsequently, the BPSK modulation technique is applied, along with the addition of a carrier wave to the modulator. Following this modulation, feature extraction is carried out from the signal. To enhance security, a MQM is applied to encrypt this feature, securing it against potential attackers, as illustrated in Fig. 2. Furthermore, the ciphered binary code is stored within a ciphered pHCI device, a process we will elucidate further later on. This dual function not only aids in preserving the patient's life but also ensures the precise dosage delivery by determining which drug molecules should be released from the liposomes. Notably, liposomes serve as nano-carriers for the information particles within the present study.
4.1.1 Modified quadratic map
A fundamental example of a chaotic system is the quadratic map. The traditional quadratic map equation is given by [23]:
where \({\varvec{r}}\in \boldsymbol{ }[1.5,\boldsymbol{ }2]\) is a parameter of the chaotic map, \({\varvec{a}}\) is constant and n is the iteration index.
The equation of the MQM is:
We supplant \({\mathbf{x}}_{\mathbf{n}}^{2}\) in Eq. (2) with the term \({\left(1-\mathbf{a}{\mathbf{x}}_{\mathbf{n}}\right)}^{2}\) and take modulo 1 division. Using MQM concluded in [20, 21] with a one unique value of a = 8 and all values except a chaotic parameter r = 0.11, 1.11 periodically to ∞, we generate the signal chaotic behavior coming from the medical staff until reaching to ciphered pHCI.
4.1.2 The Encrypted scheme
The binary code key (thermal or light) and a particular filter will be used to implement the convolution kernel algorithm. The developed algorithm is based on one-round diffusion, and the binary codes (thermal/ light) and an initial key determine its key. The stages for ciphering the code are illustrated in Fig. 3.
We can implement the new ciphered binary code in pHCI device after getting it. So, the ciphered pH-cyber can correspond to any binary code as indicated in the next phase. If a physical cyber finds the identical code, it can provide the instruction for releasing the drug particles for thermal or light; otherwise, no order can be given. So, by using this procedure, the patient's life can be saved and he can also be provided with the correct dosage.
4.2 Inside ciphered pH-cyber-interface
The authentication process is illustrated in Fig. 2, and it is performed by an encrypted pHCI to determine the appropriate therapeutic dose for release. Depending on the responsive binary code type (thermal/light), the encrypted pHCI can instruct the bio-nanosensor to initiate the ordering of liposomes for releasing their contents. Additionally, the received encrypted binary code is combined with the demodulator filter by the encrypted pHCI to generate a new binary code. This new binary code is then used in convolution with a specific encryption key to determine which dose should be released by the liposomes. The convolution results are subsequently compared to the original binary code that was previously stored within the encrypted pHCI using the Hamming distance metric. Administering the correct dosage at the precise time can potentially save the patient's life.
5 The proposed multi compartmental model based on a ciphered pH-cyber interface
Figure 4 shows a flow diagram of MC in the blood vascular (or cardiovascular) network using the proposed multi-compartment model based on the ciphered pH-cyber interface, where w1(t) and v1(t) indicate DOX molecule concentrations in the forward and reverse path, respectively. In the proposed model, we focus on delivering an accurate DOX drug to a specific tumor cell. The ciphered pH-cyber has been used to ensure the model's efficacy and maximizing the privacy. As a result, it will be able to switch between a targeted nanonetwork within the human body and the Internet domain which is according to the command signal encrypted that is been sent via medical staff to the pHCI to ensure the appropriate drug safely release inside targeted nano-network. This proposed model helps in saving the patient life and takes the appropriate dosage without any risk with privacy enhancement. The DOX drug is delivered from the tumor plasma to the tumor tissue using a coupled of ODE-PDE model based on pharmacokinetics model. Extracellular drugs diffuse inside the tumor cord, and tumor cells' absorption and efflux are governed by saturable absorption. The mortality of tumor cell is also determined by the peak intracellular drug concentration. Moreover, the DOX drug concentration in each compartment is the ratio of molecules number to compartment volume. As a result, in the following subsection, we provide an analysis of multi-compartment framework-based MC in both the forward and reverse directions. For more clarification, we provide the descriptions of the main parameters that will be used in this analysis in Table 2.
5.1 The forward compartmental direction
The forward compartmental direction is considered from ciphered pHCI to the targeted nano-network. In order to enhance DOX drug concentration and increase privacy, the following steps are performed:
-
Step 1: An encrypted command signal (in binary format) is sent over the network by medical personnel.
-
Step 2: This signal is picked up by the access point and promptly sent to a ciphered pHCI to convert the encrypted EM wave to a suitable bio-signal. After decrypting the received signal binary command, it can be used in the combinational logic circuit to provide a thermal or optical response that activates the nRT1 (PEG Liposome) and causes the DOX drugs to be released. In the context of molecular communications technology, the DOX drug as nano-particles is stored in nRT1 (liposome), which is thermal or photosensitive. In the forward path, we consider the output of the electro-bio unit \({{\varvec{g}}}^{({\varvec{f}})}\) (t) is given by:
$$\begin{array}{cc}{g}^{(f)}={\int }_{0}^{{R}_{IN}}\xi \omega \left(t\right)dt,& {\omega }_{0}={\left.\xi \omega \left(t\right)\right|}_{t={R}_{IN}}\end{array}$$(4)Where RIN is the time difference between the time of starting the injection process tA and the time of starting the release of DOX drug tR and \({\varvec{\xi}}\) infers the total number of liposomes in the system model. The concentration of DOX drug that is released at instant time t is represented by \({\varvec{\omega}}\left({\varvec{t}}\right)\). We consider \({{\varvec{\omega}}}_{0}\) is the value of \({{\varvec{g}}}^{({\varvec{f}})}\) that is required at the targeted site. The deployment of IoBNT is to control/adopt the appropriate period RIN and \({{\varvec{\upomega}}}_{0}\) to achieve the desired concentration of DOX drugs.
The nRT1 encapsulated DOX drugs are injected intravenously and it passively diffuses via the vascular network, and then it enters the targeted location in the proposed model. If the nRT1 is stimulated by photo or thermal stimulation at instant t (release time), the probability density function of DOX drug release by nRT1 is expressed as [22]:
$$\omega \left(t\right)={d}_{t}{\omega }_{R }\left(1-{e}^{-\delta t}\right)$$(5)where \({{\varvec{\upomega}}}_{{\varvec{R}}}\) infers the released DOX drug concentration, δ is the release rate and equivalent to the first-order constant rate. According to the type of the stimulation, we have two types of releasing rate for temperature or light time denoted by δt or δl, respectively, where δt, δl ∈ δ. In Eq. (2),\({{\varvec{d}}}_{{\varvec{t}}}=\frac{{\varvec{d}}}{{\varvec{d}}{\varvec{t}}}\) and \({{\varvec{\upomega}}}_{\mathbf{R}}\)≈\(\underset{0}{\overset{\boldsymbol{\infty }}{\int }}{\varvec{\upomega}}({\varvec{t}}){\varvec{d}}{\varvec{t}}\).
The in-body molecular compartment model is depicted in Fig. 5, the differential equations derived from the forward compartment model can be expressed by:
$${d}_{t}{w}_{1}\left(t\right)=-{w}_{1}\left(t\right)\left({k}_{12}+{k}_{10}\right)+{k}_{21}{w}_{3}\left(t\right)$$(6)$${d}_{t}{w}_{3}\left(t\right)={k}_{12}{w}_{1}\left(t\right)-{k}_{21}{w}_{3}\left(t\right)$$(7)where the subscripts 0, 1, and 2 indicate the position of the compartment. By considering the initial conditions \({{\varvec{w}}}_{1}\)(0) = \({{\varvec{g}}}^{({\varvec{f}})}\) and \({{\varvec{w}}}_{2}\)(0) = 0.
-
Step 3: The drug is delivered to the blood vessel via the ciphered pHCI injection storage machine, where the DOX particles are assembled and delivered to the tumor plasma, using both passive and active targeting to ensure that the treated particles remain as long as possible at targeting the required cell without being attacked by the body's immune system. The rate of change of drug concentration in the tumor plasma compartment is described by:
$${d}_{t}{{\text{w}}}_{2}\left({\text{t}}\right) =-\frac{1}{{{\text{vp}}}_{{\text{t}}}}\times {\text{ps}}\left({{\text{w}}}_{2}\left({\text{t}}\right) \times \mathrm{ UDOX}\right)+\frac{1}{{{\text{vp}}}_{{\text{t}}}}\times {\text{ps}}\left({{\text{w}}}_{4}\left({\text{t}}\right)\times {{\text{UDOX}}}_{{\text{e}}}\right)-{{\text{Fpv}}}_{{\text{t}}}\times {{\text{w}}}_{2 }\left({\text{t}}\right)+\mathrm{ Fp}{{\text{v}}}_{{\text{t}}}\times {{\text{w}}}_{1 }({\text{t}})$$(8)where the first term is the trans vascular transport by passive diffusion which is depending on the permeability surface area product ps, considering the different volumes of the tumor plasma compartment and Extravascular Extracellular space (EES). The second and third terms represent the amount of DOX drug which are transported between the plasma tumor compartment and the systemic plasma compartment by blood perfusion, respectively.
-
Step 4: The transfer of drugs from the tumor plasma compartment to the tumor tissue EES, as well as the passive diffusion of DOX, relies on the permeability surface area product ps. This takes into account variations in the volumes of the tumor plasma compartment and EES, as well as the quantity of drug being transported between the tumor plasma compartment and the systemic plasma compartment through blood perfusion. The alteration in the concentration of DOX within the tumor tissue EES is decribed by:
$${d}_{t}{{\text{w}}}_{4}\left({\text{t}}\right)= \frac{1}{{{\text{ve}}}_{{\text{t}}}}\times {\text{ps}}\left({{\text{w}}}_{2}\left({\text{t}}\right)\times {\text{UDOX}}\right)-\frac{1}{{{\text{ve}}}_{{\text{t}}}}\times {\text{ps}}\left({{\text{w}}}_{4}\left({\text{t}}\right)\times {{\text{UDOX}}}_{{\text{e}}}\right)-{{\text{k}}}_{3{{\text{w}}}_{{\text{i}}}}\times \left(\frac{{{\text{k}}}_{1{{\text{w}}}_{{\text{i}}}}\times {{\text{w}}}_{4 }\left({\text{t}}\right)\times \mathrm{ UDO}{{\text{X}}}_{{\text{e}}}+ {{\text{k}}}_{2{{\text{w}}}_{{\text{i}}}}\times {{\text{w}}}_{4 }\left({\text{t}}\right)\times \mathrm{ UDO}{{\text{X}}}_{{\text{e}}}}{\left({{\text{k}}}_{{{\text{iw}}}_{{\text{i}}}}+{{\text{w}}}_{4 }\left({\text{t}}\right)\times \mathrm{ UDO}{{\text{X}}}_{\mathrm{e }}\right)}-{{\text{k}}}_{5{{\text{w}}}_{{\text{i}}}}\times {{\text{w}}}_{5}\left({\text{t}}\right)\right)$$(9)where, \(\mathbf{U}\mathbf{D}\mathbf{O}\mathbf{X}\) and \({\mathbf{U}\mathbf{D}\mathbf{O}\mathbf{X}}_{{\varvec{e}}}\) denote the plasma binding for Dox drug and binding for Dox to proteins in EES, respectively. In Eq. (9), the first term represents the transvascular transport of Dox drug between the tumor plasma compartment and tumor EES. The second term describes the transport from tumor EES into the tumor cells.
-
Step 5: Finally, we assume two different concurrent intracellular uptake mechanisms are conducted of Dox: (1) passive diffusion across the cell membrane, and (2) an active transport mechanism, which is most likely, the drug received by the nano-transceivers in tumor tissues EES and uptake by the tumor cells (targeted cell). The rate of change of drug concentration within the tumor cells is expressed by:
$${d}_{t}{{\text{w}}}_{5}\left({\text{t}}\right) ={{\text{k}}}_{3{{\text{w}}}_{{\text{i}}}}\times \left({{\text{k}}}_{1{{\text{w}}}_{{\text{i}}}} \times {{\text{w}}}_{4}\left({\text{t}}\right)+\frac{{{\text{k}}}_{2{{\text{w}}}_{{\text{i}}}} \times {{\text{w}}}_{4}\left({\text{t}}\right)}{{({\text{k}}}_{{{\text{iw}}}_{{\text{i}}}}+{{\text{w}}}_{4}({\text{t}})) }- {{\text{k}}}_{5{{\text{w}}}_{{\text{i}}}} \times {{\text{w}}}_{5}\left({\text{t}}\right)\right)$$(10)where, the first term explains the transport from tumor EES into the tumor cells.
5.2 The reverse compartmental direction
In the reverse compartmental direction, the bio-electro unit transforms the biochemical signal picked up in the blood network by the bio-nanosensor into electrical output. Because of a lack of medication particles delivery, malfunctioning, or other reasons, a bio-nanosensor detects variations in molecular data from the targeted cell and transmits them as a protein to the targeted site via the cardiovascular system. The pH-cyber face receives the released molecular information from the bio-nanosensor and converts it to an equivalent electric signal. Furthermore, the released molecular information from bio-nanosensor may be received by the receptors of the intravascular probe or diffused to excite the cellular pathway to activate them according to computed transcription factors, as shown in Fig. 1. Based on the bio-luminescent reaction in [15], such reaction produces phosphate group (PP), adenosine monophosphate (AMP), and light hv. Thereafter, the bio-luminescence intensity, I(t) equation, including the Luciferase, (LU) and adenosine triphosphate (ATP) can be expressed according to the Michaelis–Menten mechanisms [15] as follow:
where X and L are the concentration of ATP and LU, respectively.\({\boldsymbol{\alpha }}_{{\varvec{m}}}\), \({\boldsymbol{\alpha }}_{{\varvec{L}}}\) denote the Michaelis–Menten constant and the catalytic reaction constant, respectively. The term L can be calculated using the mRNA gene differential equations [23] and the Hill function [24]. In contrast to [25, 26], where oscillatory input was the transcription factor, the activation of the transcription factor into the cellular structure is attributed to receptor-mediated or direct diffusion of information particles, which is dependent on diffusing information molecules concentration. As a result, the transcription factor concentration that describes the hypothesized pH-cyber interaction can be represented as:
where \({{\varvec{g}}}^{\left({\varvec{r}}\right)}\left({\varvec{t}}\right)\) is biological signals in the reverse direction and μ infers the signal transduction at the cellular structure surface via which the Dox medication propagate.
The concentration of Dox medication of each compartment is indicated by the ratio of the number of Dox particles to the volume of the compartment. Figure 6 describes the central compartment is a systemic plasma with the concentration of molecules denoted by \({{\varvec{v}}}_{1}\)(t), while the concentration of particles in the tumor intracellular space is denoted by \({{\varvec{v}}}_{2}\) (t). The concentration of molecules that are removed or biochemically altered over time is represented by the function \({{\varvec{w}}}_{{\varvec{e}}{\varvec{l}}}\)(t), which is a function of the elimination rate \({{\varvec{k}}}_{10}\). According to [15], this concentration consists of molecules that are phagocytosed, react, and adhere, as well as molecules that are absorbed through tissues that are not targeted and removed by the liver. The parameters \({{\varvec{k}}}_{12,{\varvec{r}}}\) and \({{\varvec{k}}}_{21,{\varvec{r}}}\), respectively, are used to represent the first-order rate constants going into or out of the targeted nano-network compartment. These rate constants are frequently influenced by the disparity in compartment concentrations, the size of the fenestra across the endothelial cell network, and the characteristics of the diffusing information particles [27]. We consider the reverse of the traditional multi-compartment model, and the rate equations are as follows:
where the term \({{\varvec{k}}}_{1}\) is the ligand-receptor binding constant and \({{\varvec{k}}}_{12,{\varvec{r}}}\) and \({{\varvec{k}}}_{21,{\varvec{r}}}\) are the kinetic rate constants.
The values t = 0, \({{\varvec{v}}}_{1}\)(0) = 0 and \({{\varvec{v}}}_{2}\)(0)=\({{\varvec{n}}}_{0}\) are considered as initial conditions. Additionally, \({{\varvec{n}}}_{0}\) is the total concentration of the molecules emitted by the bio-nanosensor. Actually, we track the different transition states of the \({{\varvec{n}}}_{0}\) via the IoBNT technology according to the expected value of I(t), that translated by the pHCI to \({{\varvec{c}}}^{\left({\varvec{r}}\right)}({\varvec{t}})\), where the value of \({{\varvec{c}}}^{\left({\varvec{r}}\right)}\left({\varvec{t}}\right)\) is either 1 for I(t) ≥ \({{\varvec{I}}}_{{\varvec{o}}}\), or 0 for I(t) <\({{\varvec{I}}}_{{\varvec{o}}}\) according to Eq. (16).
6 Simulation results and discussions
This section is comprised of two subsections. The first subsection focuses on evaluating the performance of the proposed privacy system implemented on top of the pHCI, utilizing either the light or thermal code as the communication medium. The performance metrics employed for assessment include the Receiver Operating Characteristic (ROC) curve [27] and the Equal Error Rate (EER).
The determination of whether the two binary codes (light or thermal) match is conducted using the Hamming Distance (HD). The HD is calculated as the count of non-equivalent bits between the stored and generated binary codes. For two binary codes, A and B, each consisting of N bits, the HD can be calculated as follows [28]:
where \({{\varvec{A}}}_{{\varvec{j}}}\) and \({{\varvec{B}}}_{{\varvec{j}}}\) are the ith bit of the query and the stored binary codes, respectively while N is the total number of bits in the binary codes.
The second subsection aims to delve into the performance of the proposed multi-compartmental model in conjunction with the IoBNT-based encrypted pHCI, assessed in both forward and reverse directions. For each run, we applied the default parameters, which are detailed in Table 2. Furthermore, the parameters used for each scenario were selected based on the results of previous tests conducted in [27].
6.1 The performance evaluation of proposed cipher scheme
The proposed cipher technique, employed outside the intra-body nanonetwork, relies on command signals received from medical staff by the encrypted pHCI device, employing both Error Equalization (EE) and the ROC curve [29]. The ROC curve is constructed by plotting the True Positive Rates (TPR) against the False Positive Rates (FPR), with True Negative Rates (TNR) referred as specificity and TPR as sensitivity. The False Rejection Rate (FRR), which is calculated as (1—TPR), measures the possibility of the system erroneously rejecting a correct binary code transmitted by medical staff when it is, in fact, received by the ciphered pHCI device. Conversely, the FPR gauges the likelihood of the system incorrectly accepting an attacker's binary code as the correct binary code received by the ciphered pHCI device. Consequently, based on this decision, the ciphered pHCI device either continues or ceases sending commands to the bio-nanosensors for dosing instructions. To trigger the release of contents from the liposomes for dosing purposes, a command is issued to the bio-nanosensor by the ciphered pHCI device. The following equations are utilized to calculate the Positive Predictive Value (PPV) and Negative Predictive Value (NPV) for assessing the performance of the proposed privacy scheme:
Table 1 presents the performance evaluation metrics for the considered scheme, covering two binary codes: thermal and light. As indicated in Table 1, both the thermal and light codes exhibit high sensitivity and specificity, with values of 95.333% and 100% for sensitivity and 95% and 100% for specificity, respectively. The overall performance of the proposed privacy scheme, as measured by EER, accuracy, NPV, and PPV, has been notably satisfactory. Therefore, we have effectively enhanced security over the pHCI device at a reasonable cost while safeguarding the patient's life. The ROC curve for the proposed scheme is depicted in Fig. 7a and b, when the thermal and light signals are transmitted from medical personnel to the pHCI device. It is noteworthy that the MQM technique successfully protected signals until they reached the pHCI device. Consequently, the administration of the correct dosage at the precise time has played a pivotal role in saving the patient's life.
The EER is inversely proportional to the system performance. As seen in Table 1, EER for using the thermal code is 0.2554. On the other hand, it is 0.2571 for the light code. Lower EER values indicate better recognition performance. Also, the ability to distinguish the generated and stored binary code is measured by the decidability metric \(\acute{\varvec{d}}\) [21, 30], which is computed by Eq. (20).
where \({{\varvec{\mu}}}_{\mathbf{i}}\) and \({{\varvec{\mu}}}_{{\varvec{g}}}\) are the means and \({{\varvec{\sigma}}}_{{\varvec{i}}}^{2}\) and \({{\varvec{\sigma}}}_{{\varvec{g}}}^{2}\) are the variances of the stored and generated binary codes. As shown in Table 1, the obtained values of the decidability metric for the thermal and light codes are 4.4575 and 4.3183, respectively. The larger decidability value indicates high recognition performance.
We have employed the default parameters for each plot in our analysis. Furthermore, we incorporated parameters from previous experiments conducted in [12, 13, 31, 32] to configure each scenario effectively. The performance evaluation of this study will rely on the efficacy metrics specified in Table 2.
6.2 Effects of drug concentration in forward direction
Figures 8 and 9 illustrate the impact of various parameters on the drug concentration bound for tumor cells within the multi-compartmental model, employing the ciphered pHCI. To attain the desired outcomes, we employed Eqns. (4) and (6) to (10). Figures 8 and 9 specifically present the porosity values plotted against time. Additionally, Table 2 provides a comprehensive description of the simulation parameters and their respective values utilized in this investigation.
Drug concentration varying in \({{\varvec{w}}}_{2}\left({\varvec{t}}\right),{{\varvec{w}}}_{4}\left({\varvec{t}}\right) and {{\varvec{w}}}_{5}\left({\varvec{t}}\right)\) delivered to the intrabody nano-network with (a) \({{\varvec{\omega}}}_{0}\), b \({{\varvec{k}}}_{10}\), c–d \({{\varvec{k}}}_{10\boldsymbol{ }}{\text{and}}{\boldsymbol{ }{\varvec{\omega}}}_{0}\), e \({{\varvec{k}}}_{12,}\), f \({{\varvec{k}}}_{12}\) and \({{\varvec{\omega}}}_{0}\)
Drug concentration varying in \({{\varvec{w}}}_{2}\left({\varvec{t}}\right),{{\varvec{w}}}_{4}\left({\varvec{t}}\right)\boldsymbol{ }{\text{and}}\boldsymbol{ }{{\varvec{w}}}_{5}\left({\varvec{t}}\right)\) delivered to the intrabody nano-network with (a), b, c, and (d) Cell uptake parameters and their effect on \({{\varvec{w}}}_{5}\left({\varvec{t}}\right)\), e effect of ps on \({{\varvec{w}}}_{5}\left({\varvec{t}}\right),\) f effect of blood perfusion \({{\varvec{w}}}_{0}\)
As illustrated in Fig. 8a, the fraction of injected \({{\varvec{\omega}}}_{0}\), which is \({{\varvec{g}}}^{({\varvec{f}})}\) is determined by the liposome release rate δ and the period \({{\varvec{R}}}_{\mathbf{I}\mathbf{N}}\) among the time of injection and the start of molecular released. The consequences of altering injected molecules \({{\varvec{\omega}}}_{0}\) from the ciphered pHCI packed by liposome and propagated across the blood vessel network \({{\varvec{w}}}_{1}({\varvec{t}})\) are depicts in Fig. 8a. Furthermore, Fig. 8a shows that a larger concentration of emitted particles increases the concentration of medication particles around the reception region of the targeted cell \({{\varvec{w}}}_{5}\left({\varvec{t}}\right)\) inside the targeted nano-network \({(\mathrm{targeted tissue}) {\varvec{w}}}_{4}({\varvec{t}})\). This means that a released molecule produced efficiently reach nRT2 as drug particles and increases their binding to the targeted cells before reaching nRT3 as information particles which eliminate the dose.
In Fig. 8b, c, d, we can see how the difference in the elimination rate \({{\varvec{k}}}_{10}\), impacts the results. Based on these figures, these data show that \({{\varvec{k}}}_{10}\) influences both the concentration of drug particles attached to diseased cells \({{\varvec{w}}}_{5}({\varvec{t}})\) and, invariably, IoBNT. This indicates that an increase value of this parameter causes faster clearance, reducing both the raises plasma concentration and duration of the effects. As a result, \({{\varvec{k}}}_{10}\) is a critical parameter to be considered when developing IoBNT. Figure 8e and f demonstrate how a larger constant forward rate \({{\varvec{k}}}_{12}\) raises the concentration of drug particles in the reception region within the targeted tissues \({{\varvec{w}}}_{4}\left({\varvec{t}}\right).\) Furthermore, Fig. 8e and f reveal that the compartment differential, the scale of the fenestra that connects the nano-network to the blood network via the endothelial cell network, and the features of the diffusing information particles all have an effect on this parameter.
In addition, we analyze the efficacy of the proposed multi-compartmental model based on ciphered pHCI to demonstrate the effect of forward cell uptake parameters \({\mathbf{k}}_{1{\mathbf{w}}_{\mathbf{i}}},{\mathbf{k}}_{3{\mathbf{w}}_{\mathbf{i}}}\) inside the tumor cell. Table 2 shows the values of the parameters in the multi compartmental model that we utilized. The effect of the forward rate constants \({\mathbf{k}}_{1{\mathbf{w}}_{\mathbf{i}}},{\mathbf{k}}_{3{\mathbf{w}}_{\mathbf{i}}},\) permeability surface area product ps, and plasma flow Fpv_t on \({{\varvec{w}}}_{5}({\varvec{t}})\), which are dependent on blood perfusion \({{\varvec{w}}}_{0}\) and blood volume in liver parenchyma BV are shown in Fig. 9a, b, c, d, e, and f, respectively.
Figure 9a, b, c, and d depict the effect of forward cell uptake parameters \({\mathbf{k}}_{1{\mathbf{w}}_{\mathbf{i}}},{\mathbf{k}}_{3{\mathbf{w}}_{\mathbf{i}}}\) inside the tumor cell (targeted cell) w5(t). Cellular absorption of molecules and drug carriers are poor in the absence of exogenous assistance. The development of potent delivery mechanisms is required for effective intracellular administration of chemic substances. As a result, an IoBNT influenced multi compartmental model was employed to improve the system's efficiency in delivering drug to specific cells in an effective and safe manner w5(t). This will help to improve the efficiency of drug uptake and transfer it to the targeted cell, as illustrated in Fig. 9a, b, c, and d, respectively. Increasing \({{\varvec{k}}}_{1{{\varvec{w}}}_{{\varvec{i}}}},{{\varvec{k}}}_{3{{\varvec{w}}}_{{\varvec{i}}}}\) obviously results in a higher value of target cell w5(t) and thus the drug concentration increases in tumor cell w5(t).
Figure 9e showcases the impact of the permeability surface area product ps on tumor cells (specifically, targeted cells), denoted as w5(t). In this context, ps signifies the capacity of a blood vessel wall to facilitate the passage of small particles, such as drugs, to the targeted cells. The study of permeability surface area is of paramount importance since the vascular wall serves as a barrier preventing large molecules from entering tumors. Moreover, Fig. 9e demonstrates that a larger ps value enhances the transport of medication to the targeted cell. Consequently, the concentration of drug particles within the infected cell increases, enabling more effective treatment. Consequently, when designing IoBNT systems, the consideration of ps becomes a crucial factor.
Figure 9f depicts the effect of w0 on plasma flow, which plays an important function in boosting the concentration of information conveyed from the ciphered pHCI to artificial machines within the targeted tissue \({{\varvec{w}}}_{4}\)(t), resulting in a stronger effect in drug delivery to the targeted cell \({{\varvec{w}}}_{5}\)(t). Furthermore, as w0 increases, the concentration of particles within the targeted cell increases to its maximum. The model's job in this scenario is to demonstrate the efficacy and influence of w0 on molecule concentration. As a result, w0 is a critical parameter to be considered when developing IoBNT.
6.3 Normalized concentration in reverse direction
To obtain the results depicted in Fig. 10, we employed Eqns. (12), (13), (14), and (15). These results illustrate the impact of various parameters on the normalized bioluminescence intensity, which serves as the source of molecular information for the ciphered pHCI in the reverse direction within the proposed multi-compartmental model. In this endeavor, we focused on refining the evaluation parameters previously utilized in [15].
The parameters values in the proposed multi compartmental model are:\({k}_{21}\)= 7.052e−5\({{\text{min}}}^{-1}\), \({k}_{12}\)=9.4e−3 \({{\text{min}}}^{-1}\) and \({k}_{10}\)= 2.1e−3 \({{\text{min}}}^{-1}\)[36]; \({k}_{12,r}\)= 0.103\(\times {10}^{-2}{{\text{min}}}^{-1}\), \({k}_{21,r}\)= 0.373\({{\text{min}}}^{-1}\); and \({k}_{1}\) = 0.1\(\times {10}^{-2}{{\text{min}}}^{-1}\). We set \(\mu\) = 1 by assuming that Dox drug molecules diffuse directly into the bio-electro transducer unit. The following factors for expressing LU are: the rate constant of translating mRNA into LU \(, {k}_{r} = 0.1\times {10}^{2}\) and \({k}_{p} = 1.5\times {10}^{2}{{\text{h}}}^{-1}\) [37], the degradation rate of mRNA is \({\gamma }_{r}= 0.1005\times {10}^{2}{{\text{h}}}^{-1}\) and the degradation rate of LU is \({\gamma }_{p}= 0.0415{\times {10}^{2}\mathrm{ h}}^{-1}\) [38]. For the bio-luminescence reaction, values of the factors utilized are, \({\alpha }_{M} = 15 \mu {\text{M}},{\alpha }_{l} =0.044\) and \({a}_{tp} =0.04\times {10}^{3}\mu {\text{L}}\)[39]. In the simulation, the noise variance applied is 0.5 \(\times {10}^{-1}\mu {\text{M}}\)[40].
Furthermore, we assume that the concentration of DOX drug molecules, denoted as \({{\varvec{n}}}_{{\varvec{i}}}\left({\varvec{t}}\right)\mathrm{ and }{{\varvec{w}}}_{{\varvec{i}}}\left({\varvec{t}}\right)\), in the presence of Gaussian noise follows a normal distribution with parameters, (0,\({{\varvec{\sigma}}}_{2}\)), where \({{\varvec{\sigma}}}_{2}\) represents the variance [41]. When analyzing the bio-luminescence intensity, we consider the wavelength of the emitted light to be relatively constant, which is suitable when the light beam remains relatively stable. Therefore, the sensitivity of the photoresistor in the pHCI is primarily influenced by the emitted light's intensity. For our simulation, we employ a value of \({\gamma }_{l} = 1.04 {{\text{e}}}^{-4}\) min-1, which is derived from experimental data found in [42], specifically concerning the exposure of liposomes to ultraviolet light (UV). In our simulation, we use used \({\gamma }_{t}\)= 0.0078 min−1 [43], a value obtained by fitting an optimal curve to experimental data collected at 42°C through the non-linear least square’s method [44]. To explore the impact of varying values such as \({{\varvec{n}}}_{0},{{\varvec{k}}}_{21,{\varvec{r}}},{{\varvec{k}}}_{1}, {\boldsymbol{\alpha }}_{{\varvec{M}}},{{\varvec{a}}}_{{\varvec{t}}{\varvec{p}}}{\text{and}}{ {\varvec{k}}}_{10}\) on the bio-luminescence density I(t), expressed in arbitrary units (a.u), which is crucial for facilitating communication between the pHCI and medical personnel, shown in Fig. 10.
In the reverse direction, the results are obtained according to the derived equations. On the normalized scale, we assume that \({I}_{0}\) = 0.007 \(\times {10}^{2}.\) A low value of \({{\varvec{n}}}_{0}\) (for example, less than 8M) is sufficient to send the electrical signal through the ciphered pHCI as shown in Fig. 10a. As a result, the bio-nanosensor must be able to release a large concentration of molecular information to be accepted effectively at the ciphered pHCI to increase the amount of concentration, n0 or we suggest using more than one bio-nanosensor.
The variation in the forward rate constant \({{\varvec{k}}}_{21,{\varvec{r}}}\) has a substantial effect on bio-luminescence intensity, as shown in Fig. 10b. As previously declared in the reverse direction, the scale of the fenestra in endothelia cell that regulates the transmission between the bloodstream and the targeted tissues effects on \({{\varvec{k}}}_{21,{\varvec{r}}}\). As well as the potentials of the diffusing molecular information, and the concentration difference between compartment \({{\varvec{v}}}_{2}\) and \({v}_{1}\), have strong effect on \({{\varvec{k}}}_{21,{\varvec{r}}}\) parameter. When \({{\varvec{k}}}_{21,{\varvec{r}}}\) increases, the ciphered pHCI receives more molecular information, which results in increased bio-luminescence, as illustrated in Fig. 10b. The bio-luminescence intensity increases significantly with the rate where the molecular information is observed by the pHCI, as illustrated in Fig. 10c. This rate is determined by the receptor, which depends on the concentration of probes or the distribution of the porous membrane through which molecular information passes across the pHCI.
Based on the preceding discussions and thorough examination of the results presented in Figs. 7, 8, 9, and 10, it can be deduced that our proposed model surpasses the model presented in [15]. This superiority can be attributed to the implementation of our proposed privacy strategy, which operates on top of the ciphered pHCI within the multi-compartmental model. This strategy enables the precise delivery of the required dosage directly to the targeted site, potentially playing a pivotal role in saving a patient's life. Furthermore, our proposed model enables the achievement of high concentrations of medication molecules in close proximity to diseased cells, ensuring rapid absorption by the targeted cells. As a result, when employing a TTDS based on IoBNT principles, our model has the potential to minimize side effects in the vicinity of healthy cells.
7 Conclusion
The IoBNT paradigm introduces a privacy system that operates on top of the pHCI. This proposed strategy comprises two integral steps. The first step aims to generate ciphered feature extraction from the signal, convolved with a mask and a specific kernel produced by the chaotic system. The utilization of an MQM technique not only enhances the secrecy of the key space but also strengthens the resilience of the ciphered features. The second step employs a multi-compartmental model to explore the consequences and variations in medication concentration concerning diseased cells, specifically tumor cells, within the intra-body nanonetwork. The results demonstrate that alterations in the system design parameters, both in the forward and reverse directions, exert a significant influence on drug concentration delivery. A comprehensive simulation campaign has been conducted to validate the proposed scheme's analysis and numerical results. The present findings establish that the compartmental model, crafted on the principles of IoBNT, holds the potential to enhance the delivery of medication molecules to targeted cells while concurrently mitigating side effects and bolstering privacy.
In the long term, our overarching goal is to leverage cutting-edge encryption technology to secure IoBNT molecular information, particularly in the presence of cryptographic keys. Consequently, we are actively exploring the integration of blockchain technology within the realm of biology to further fortify IoBNT systems. Moreover, we are planning to conduct clinical trials aimed at evaluating the safety and efficacy of IoBNT-based drug delivery systems.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Abbreviations
- AMP:
-
Adenosine monophosphate
- ATP:
-
Adenosine triphosphate
- BPSK:
-
Binary Phase Shift Keying
- Dox:
-
Doxorubicin
- EE:
-
Error Equalization
- EER:
-
Equal Error Rate
- EES:
-
Extravascular Extracellular Space
- FPR:
-
False Positive Rate
- FRR:
-
False Rejection Rate
- HD:
-
Hamming Distance
- ICT:
-
Information Communication Technology
- IoBNT:
-
Internet of Bio-Nano Thing
- IoTs:
-
Internet of Things
- LU:
-
Luciferase
- pHCI:
-
Physical Cyber Interface
- MQM:
-
Modified Quadratic Map
- NPs:
-
Nanoparticles
- MC:
-
Molecular Communication
- TDDS:
-
Targeted Drug Delivery Systems
- NPV:
-
Negative Predictive Value
- PP:
-
Phosphate group
- PPV:
-
Positive Predictive Value
- ROC:
-
Receiver Operating Characteristic curve
- TNR:
-
True Negative Rates
- TPR:
-
True Positive Rates
References
Akyildiz I, Brunetti F, Blázquez C (2008) Nanonetworks: a new communication paradigm. Comput Netw 52(12):2260–2279
Nakano T, Suda T, Okaie Y, Moore M, Vasilakos A (2014) Molecular communication among biological nanomachines: A layered architecture and research issues. IEEE Trans NanoBio 13(3):169–197
El-Kareh AW, Secomb TW (2005) Two-mechanism peak concentration model for cellular pharmacodynamics of doxorubicin. Neoplasia 7(7):705–713
Abd El-atty SM (2020) Health monitoring scheme-based FRET nanocommunications in internet of biological nanothings. Int J of Comm Sys 33(11):1–17
Jiang Y, Yin S, Dong J, Kaynak O (2021) A review on soft sensors for monitoring, control and optimization of industrial processes. IEEE Sensors J 21(11):12868–12881
Kuscu M, Unluturk BD (2021) Internet of Bio-Nano things: a review of applications, enabling technologies and key challenges. ITU J Future Evolving Technol 2(3):1–24
Abd El-atty SM, Araf NA, Abouelazm A, Alfarraj O, Lizos KA, Shawki F (2022) Performance analysis of an artificial intelligence nanosystem with biological internet of Nano Thing. Comput Model Eng Sci. https://doi.org/10.32604/cmes.2022.020793
Chahibi Y, Pierobon M, Song SO, Akyildiz IF (2013) A molecular communication system model for particulate drug delivery systems. IEEE Trans Biomed Eng 60(12):3468–3483
Chude-Okonkwo UAK (2014) Diffusion-controlled enzyme-catalyzed molecular communication systems for targeted drug delivery. IEEE Global Communication Conference, Austin, Texas, Dec. 8–12
El-Fatyany A, Wang H, Abd El-atty SM (2021) Efficient Framework analysis for targeted drug delivery based on internet of BioNanoThings. Arab J Sci Eng 46:9965–9980 (Springer)
Chahibi Y, Pierobon M, Akyildiz I (2015) Pharmacokinetic modeling and biodistribution estimation through the molecular communication paradigm. IEEE Trans Biomed Eng 62(10):2410–2420
Chude-Okonkwo UAK, Malekian R, Maharaj BT (2016) Molecular communication model for targeted drug delivery in multiple disease sites with diversely expressed enzymes. IEEE Trans Nanobio 15(3):230–245
Al-Zubi MM, Mohan AS, Plapper P, Ling SH (2022) Intrabody molecular communication via blood-tissue barrier for internet of Bio-Nano Things. IEEE Internet Things J 9(21):21802
Felicetti L, Femminella M, Reali G, Liò P (2016) Applications of molecular communications to medicine: a survey. NanoCommunication Netw 7:27–45
Chude-Okonkwo UA, Malekian R, Maharaj B (2016) Biologically inspired bio-cyberinterface architecture and model for internet of bio-nano thingsapplications. IEEE Trans Commun 64(8):3444–3455
Lee C, Koo BH, Chae CB, Schober R (2023) The internet of bio-nano things in blood vessels. System design and prototype. J Commun Netw 25(2):222–231
Zhao NA, Martin CW, Mixson AJ (2018) Advances in delivery systems for doxorubicin. J NanomedNanotechnol 9(5):519
Ma L, Kohli M, Smith A (2013) Nanoparticles for combination drug therapy. ACS Nano 7(11):9518–9525
McKay W, Gusztak R, Bolton T, Frost A, Wang A, Kushneriuk B, Cowen J, Chen D (2015) Development of a painless injection device applying pressure, vibration, and temperature, 36th Annual Scientific Meeting of the Canadian Pain Society, Charlottetown, Canada, May 20–23
Moreira FJS (1992) Chaotic dynamics of quadratic maps. Master’s thesis, University of Porto
Abd El-Hameed HA, Ramadan N, El-Shafai W, Khalaf AAM, Ahmed HH, Elkhamy SE, Abd El-Samie FE (2022) Cancelable biometric security system based on advanced chaoticmaps. Vis Compute 38:2171–2187
Afadzi M, Davies C and Hansen YH (2010) Ultrasound stimulated release of liposomal calcein. IEEE Conference on Ultrasonics Symposium (IUS), San Diego, California USA: 11–14
Dollard MA, Billard P (2003) Whole-cell bacterial sensors for the monitoring of phosphatebioavailability. J Microbiol Methods 55(1):221–229
Yagil G, Yagil E (1971) On the relation between effector concentration and the rate of induced enzyme synthesis. Biophys J 11(1):11–27
El-atty SM, El-Taweel A, El-Rabaie S (2018) Transmission of nanoscale information based neural communication-aware ligand–receptor interactions. Neural Comput Appl 30(11):3509–3522
Vijayaraghavana R, Islam SK, Zhang M, Caylor S, Bull ND, Moser S, Terry SC, Blalock BJ, Sayler GS (2007) A bioreporter bioluminescent integrated circuit for very low-levelchemical sensing in both gas and liquid environments. Sens Actuators B Chem 123(2):922–928
Klein BG (2013) Cunnigham’s Textbook of Veterinary Physiology, Missouri. Elsevier Saunders, USA, p 226
Jain AK, Li SZ (2005) Handbook of face recognition, 1st edn. Springer
Muschelli J (2020) ROC and AUC with a binary predictor: a potentially misleading metric. J Classif 37:696–708
Ouda O, Tsumura N, Nakaguchi T (2011) On the security of BioEncoding based cancelable biometrics. IEICE Trans Inf Sys 94-D(9):768–1777
Matyka M, Khalili A, Koza Z (2008) Tortuosity-porosity relation in porous media flow. Phys Rev E 78(2):026306
ChahibiYI AIF, Balasubramaniam S, Koucheryavy YY (2015) Molecular communication modeling of antibody-mediated drug deliverysystems. IEEE Trans Biomed Eng 62(7):1683–1695
Jackson TL (2003) Intracellular accumulation and mechanism of action of doxorubicin in a spatio-temporal tumor model. J Theor Biol 220(2):201–213
Yuan F, Leunig M, Berk DA, Jain RK (1993) Microvascular permeability of albumin, vascular surface area, and vascular volume measured in human adenocarcinoma LS174T using dorsal chamber in SCID mice. Microvasc Res 45(3):269–289
Brizel DM, Klitzman B, Cook JM, Edwards J (1993) A comparison of tumor and normal tissue microvascular hematocrits and red cell fluxes in a rat window chamber model. Bio Phys 25(2):269–289
Cascone S, Lamberti G, Titomanlio G, Piazza O (2013) Pharmacokinetics of Remifentanil: a three-compartmental modeling approach. Transl Med@ UniSa 7(4):18–22
Austin CM, Stoy W, Su P, Harber MC, Bardill JP, Hammer BK, Forest CR (2014) Modeling and validation of autoinducer-mediated bacterial gene expression in microfluidic environments. Biomicrofluidics 8(3):034116
Moon TS, Lou C, Tamsir A, Stanton BC, Voigt CA (2012) Genetic programs constructed from layered logic gates in single cells. Nature 491(7423):249–253
Niwa K, Ichino Y, Kumata S, Nakajima Y, Hiraishi Y, Kato D, Viviani VR, Ohmiya Y (2010) Quantum yields and kinetics of the firefly bioluminescence reaction of beetle luciferases. Photochem Photobiol 86(5):1046–1049
Bicen AO, Akyildiz IF (2014) End-to-end propagation noise and memory analysis for molecular communication over microfluidic channels. IEEE Trans Commun 62(7):2432–2443
Bonate P (2001) Advanced methods of pharmacokinetic and pharmacodynamic systems analysis. IDrugs Investig Drugs J 4(9):1017–1020
Sharma NK, Kumar V (2015) Release kinetics of novel photosensitive liposome for triggered delivery of entrapped drug. PharmTech 8(1):106–113
Zhan W, Xu XY (2013) A mathematical model for thermosensitive liposomal delivery of Doxorubicin to solid tumor. J Drug Deliv 2013:172529
Tagami T, Ernsting MJ, Li SD (2011) Optimization of a novel and improved thermosensitive liposome formulated with DPPC and a Brij surfactant using a robust in vitro system. J Control Release 154(3):290–297
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they do not have conflict of interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kamal, I.R., El‐Atty, S.M.A., El-Zoghdy, S.F. et al. Internet of Bio-NanoThings privacy: securing a multi compartmental targeted cancer drug delivery scheme. Multimed Tools Appl (2024). https://doi.org/10.1007/s11042-024-18423-5
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11042-024-18423-5