Abstract
Background
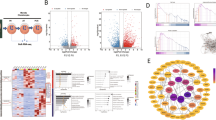
MicroRNAs (miRNAs) play critical roles in the osteogenic differentiation of human bone mesenchymal stem cells (hBMSCs), but the mechanism by which miRNAs indirectly modulate osteogenesis remains unclear. Here, we explored the mechanism by which miRNAs indirectly modulate gene expression through histone demethylases to promote bone regeneration.
Methods and results
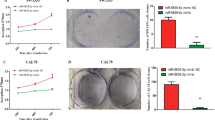
Bioinformatics analysis was performed on hBMSCs after 7 days of osteogenic induction. The differentially expressed miRNAs were screened, and potential target mRNAs were identified. To determine the bioactivity and stemness of hBMSCs and their potential for bone repair, we performed wound healing, Cell Counting Kit-8 (CCK-8), real-time reverse transcription quantitative polymerase chain reaction (RT‒qPCR), alkaline phosphatase activity, alizarin red S (ARS) staining and radiological and histological analyses on SD rats with calvarial bone defects. Additionally, a dual-luciferase reporter assay was utilized to investigate the interaction between miR-26b-5p and ten-eleven translocation 3 (TET3) in human embryonic kidney 293T cells. The in vitro and in vivo results suggested that miR-26b-5p effectively promoted the migration, proliferation and osteogenic differentiation of hBMSCs, as well as the bone reconstruction of calvarial defects in SD rats. Mechanistically, miR-26b-5p bound to the 3’ untranslated region of TET3 mRNA to mediate gene silencing.
Conclusions
MiR-26b-5p downregulated the expression of TET3 to increase the osteogenic differentiation of hBMSCs and bone repair in rat calvarial defects. MiR-26b-5p/TET3 crosstalk might be useful in large-scale critical bone defects.








Similar content being viewed by others
Data availability
Data is provided within the manuscript or supplementary information files.
References
Salhotra A, Shah HN, Levi B, Longaker MT (2020) Mechanisms of bone development and repair. Nat Rev Mol Cell Bio 21(11):696–711. https://doi.org/10.1038/s41580-020-00279-w
Doi K, Kawakami F, Hiura Y, Oda T, Sakai K, Kawai S (1995) One-stage treatment of infected bone defects of the tibia with skin loss by free vascularized osteocutaneous grafts. Microsurgery 16(10):704–712. https://doi.org/10.1002/micr.1920161009
Kengelbach-Weigand A, Thielen C, Bäuerle T, Götzl R, Gerber T, Körner C, Beier JP, Horch RE, Boos AM (2021) Personalized medicine for reconstruction of critical-size bone defects – a translational approach with customizable vascularized bone tissue. NPJ Regen Med 6(1):49. https://doi.org/10.1038/s41536-021-00158-8
Petrella G, Tosi D, Pantaleoni F, Adani R (2021) Vascularized bone grafts for post-traumatic defects in the upper extremity. Arch Plast Surg 48(01):84–90. https://doi.org/10.5999/aps.2020.00969
Bello SA, Cruz-Lebrón J, Rodríguez-Rivera OA, Nicolau E (2023) Bioactive scaffolds as a promising alternative for enhancing critical-size bone defect regeneration in the Craniomaxillofacial Region. ACS Appl Bio Mater 6(11):4465–4503. https://doi.org/10.1021/acsabm.3c00432
Nakagawa S, Ando W, Shimomura K, Hart DA, Hanai H, Jacob G, Chijimatsu R, Yarimitu S, Fujie H, Okada S, Tsumaki N, Nakamura N (2023) Repair of osteochondral defects: efficacy of a tissue-engineered hybrid implant containing both human MSC and human iPSC-cartilaginous particles. NPJ Regen Med 8(1):59. https://doi.org/10.1038/s41536-023-00335-x
Chai S, Huang J, Mahmut A, Wang B, Yao Y, Zhang X, Zhuang Z, Xie C, Xu Z, Jiang Q (2022) Injectable photo-crosslinked bioactive BMSCs-BMP2-GelMA scaffolds for bone defect repair. Front Bioeng Biotech 10:875363. https://doi.org/10.3389/fbioe.2022.875363
Hensley AP, McAlinden A (2021) The role of microRNAs in bone development. Bone 143:115760. https://doi.org/10.1016/j.bone.2020.115760
Greer EL, Shi Y (2012) Histone methylation: a dynamic mark in health, disease and inheritance. Nat Rev Genet 13(5):343–357. https://doi.org/10.1038/nrg3173
Gordon JAR, Stein JL, Westendorf JJ, van Wijnen AJ (2015) Chromatin modifiers and histone modifications in bone formation, regeneration, and therapeutic intervention for bone-related disease. Bone 81:739–745. https://doi.org/10.1016/j.bone.2015.03.011
O’Brien J, Hayder H, Zayed Y, Peng C (2018) Overview of MicroRNA biogenesis, mechanisms of actions, and circulation. Front Endocrinol 9:402. https://doi.org/10.3389/fendo.2018.00402
Incoronato M, Urso L, Portela A, Laukkanen MO, Soini Y, Quintavalle C, Keller S, Esteller M, Condorelli G (2011) Epigenetic regulation of miR-212 expression in lung cancer. PLoS ONE 6(11):e27722. https://doi.org/10.1371/journal.pone.0027722
Ke X, Qu Y, Rostad K, Li W, Lin B, Halvorsen OJ, Haukaas SA, Jonassen I, Petersen K, Goldfinger N, Rotter V, Akslen LA, Oyan AM, Kalland K-H (2009) Genome-wide profiling of histone H3 lysine 4 and lysine 27 trimethylation reveals an epigenetic signature in prostate carcinogenesis. PLoS ONE 4(3):e4687. https://doi.org/10.1371/journal.pone.0004687
Ryu S, McDonnell K, Choi H, Gao D, Hahn M, Joshi N, Park S-M, Catena R, Do Y, Brazin J, Vahdat Linda T, Silver Randi B, Mittal V (2013) Suppression of miRNA-708 by polycomb group promotes metastases by calcium-induced cell migration. Cancer Cell 23(1):63–76. https://doi.org/10.1016/j.ccr.2012.11.019
Suzuki H, Maruyama R, Yamamoto E, Kai M (2013) Epigenetic alteration and microRNA dysregulation in cancer. Front Genet 4:258. https://doi.org/10.3389/fgene.2013.00258
Wang B, Liu Y, Luo F, Xu Y, Qin Y, Lu X, Xu W, Shi L, Liu Q, Xiang Q (2016) Epigenetic silencing of microRNA-218 via EZH2-mediated H3K27 trimethylation is involved in malignant transformation of HBE cells induced by cigarette smoke extract. Arch Toxicol 90(2):449–461. https://doi.org/10.1007/s00204-014-1435-z
Lu X, Zhang Y, Zheng Y, Chen B (2021) The miRNA-15b/USP7/KDM6B axis engages in the initiation of osteoporosis by modulating osteoblast differentiation and autophagy. J Cell Mol Med 25(4):2069–2081. https://doi.org/10.1111/jcmm.16139
Tang Y, Zhang L, Tu T, Li Y, Murray D, Tu Q, Chen JJ (2018) MicroRNA-99a is a novel regulator of KDM6B-mediated osteogenic differentiation of BMSCs. J Cell Mol Med 22(4):2162–2176. https://doi.org/10.1111/jcmm.13490
Zheng H, Wang N, Li L, Ge L, Jia H, Fan Z (2021) Mir-140-3p enhanced the osteo/odontogenic differentiation of DPSCs via inhibiting KMT5B under hypoxia condition. Int J Oral Sci 13(1):41. https://doi.org/10.1038/s41368-021-00148-y
Qu L, Yin T, Zhao Y, Lv W, Liu Z, Chen C, Liu K, Shan S, Zhou R, Li X, Dong H (2023) Histone demethylases in the regulation of immunity and inflammation. Cell Death Discov 9(1):188. https://doi.org/10.1038/s41420-023-01489-9
Lee S-H, Kim O, Kim H-J, Hwangbo C, Lee J-H (2021) Epigenetic regulation of TGF-β-induced EMT by JMJD3/KDM6B histone H3K27 demethylase. Oncogenesis 10(2):17. https://doi.org/10.1038/s41389-021-00307-0
Abu-Hanna J, Patel JA, Anastasakis E, Cohen R, Clapp LH, Loizidou M, Eddama MMR (2022) Therapeutic potential of inhibiting histone 3 lysine 27 demethylases: a review of the literature. Clin Epigenetics 14(1):98. https://doi.org/10.1186/s13148-022-01305-8
Xu J, Yu B, Hong C, Wang C-Y (2013) KDM6B epigenetically regulates odontogenic differentiation of dental mesenchymal stem cells. Int J Oral Sci 5(4):200–205. https://doi.org/10.1038/ijos.2013.77
Liu D, Wang Y, Jia Z, Wang L, Wang J, Yang D, Song J, Wang S, Fan Z (2015) Demethylation of IGFBP5 by histone demethylase KDM6B promotes mesenchymal stem cell-mediated periodontal tissue regeneration by enhancing osteogenic differentiation and anti-inflammation potentials. Stem Cells 33(8):2523–2536. https://doi.org/10.1002/stem.2018
Ye L, Fan Z, Yu B, Chang J, Al Hezaimi K, Zhou X, Park N-H, Wang C-Y (2018) Histone demethylases KDM4B and KDM6B promotes osteogenic differentiation of human MSCs. Cell Stem Cell 23(6):898–899. https://doi.org/10.1016/j.stem.2018.11.002
Han X, Fan Z (2021) MicroRNAs regulation in osteogenic differentiation of mesenchymal stem cells. Front Dent Med 2:747068. https://doi.org/10.3389/fdmed.2021.747068
Foessl I, Kotzbeck P, Obermayer-Pietsch B (2019) miRNAs as novel biomarkers for bone related diseases. J Lab Precis Med 4. https://doi.org/10.21037/jlpm.2018.12.06
Valenti MT, Dalle Carbonare L, Mottes M (2018) Role of microRNAs in progenitor cell commitment and osteogenic differentiation in health and disease (review). Int J Mol Med 41(5):2441–2449. https://doi.org/10.3892/ijmm.2018.3452
Ye W, Yang Z, Cao F, Li H, Zhao T, Zhang H, Zhang Z, Yang S, Zhu J, Liu Z, Zheng J, Liu H, Ma G, Guo Q, Wang X (2022) Articular cartilage reconstruction with TGF-β1-simulating self-assembling peptide hydrogel-based composite scaffold. Acta Biomater 146:94–106. https://doi.org/10.1016/j.actbio.2022.05.012
Yang S, Wang C, Zhu J, Lu C, Li H, Chen F, Lu J, Zhang Z, Yan X, Zhao H, Sun X, Zhao L, Liang J, Wang Y, Peng J, Wang X (2020) Self-assembling peptide hydrogels functionalized with LN- and BDNF- mimicking epitopes synergistically enhance peripheral nerve regeneration. Theranostics 10(18):8227–8249. https://doi.org/10.7150/thno.44276
Lu J, Shen X, Sun X, Yin H, Yang S, Lu C, Wang Y, Liu Y, Huang Y, Yang Z, Dong X, Wang C, Guo Q, Zhao L, Sun X, Lu S, Mikos AG, Peng J, Wang X (2018) Increased recruitment of endogenous stem cells and chondrogenic differentiation by a composite scaffold containing bone marrow homing peptide for cartilage regeneration. Theranostics 8(18):5039–5058. https://doi.org/10.7150/thno.26981
Zhu J, Yang S, Qi Y, Gong Z, Zhang H, Liang K, Shen P, Huang Y-Y, Zhang Z, Ye W, Yue L, Fan S, Shen S, Mikos AG, Wang X, Fang X (2022) Stem cell–homing hydrogel-based miR-29b-5p delivery promotes cartilage regeneration by suppressing senescence in an osteoarthritis rat model. Sci Adv 8(13):eabk0011. https://doi.org/10.1126/sciadv.abk0011
Han X, Yang R, Yang H, Cao Y, Han N, Zhang C, Shi R, Zhang Z, Fan Z (2020) miR-4651 inhibits cell proliferation of gingival mesenchymal stem cells by inhibiting HMGA2 under nifedipine treatment. Int J Oral Sci 12(1):10. https://doi.org/10.1038/s41368-020-0076-8
Yang H, Cao Y, Zhang J, Liang Y, Su X, Zhang C, Liu H, Han X, Ge L, Fan Z (2020) DLX5 and HOXC8 enhance the chondrogenic differentiation potential of stem cells from apical papilla via LINC01013. Stem Cell Res Ther 11(1):271. https://doi.org/10.1186/s13287-020-01791-8
Montibus B, Cercy J, Bouschet T, Charras A, Maupetit-Méhouas S, Nury D, Gonthier-Guéret C, Chauveau S, Allegre N, Chariau C, Hong CC, Vaillant I, Marques CJ, Court F, Arnaud P (2021) TET3 controls the expression of the H3K27me3 demethylase Kdm6b during neural commitment. Cell Mol Life Sci 78(2):757–768. https://doi.org/10.1007/s00018-020-03541-8
Hansen MS, Madsen K, Price M, Søe K, Omata Y, Zaiss MM, Gorvin CM, Frost M, Rauch A (2024) Transcriptional reprogramming during human osteoclast differentiation identifies regulators of osteoclast activity. Bone Res 12(1):5. https://doi.org/10.1038/s41413-023-00312-6
Wang J, Liu S, Li J, Zhao S, Yi Z (2019) Roles for miRNAs in osteogenic differentiation of bone marrow mesenchymal stem cells. Stem Cell Res Ther 10(1):197. https://doi.org/10.1186/s13287-019-1309-7
Wei F, Yang S, Guo Q, Zhang X, Ren D, Lv T, Xu X (2017) MicroRNA-21 regulates osteogenic differentiation of periodontal ligament stem cells by targeting Smad5. Sci Rep 7(1):16608. https://doi.org/10.1038/s41598-017-16720-8
Ma J, Lin Y, Zhu J, Huang K, Wang Y (2021) MiR-26b-5p regulates the preadipocyte differentiation by targeting FGF21 in goats. Vitro Cell Dev Biol Anim 57(3):257–263. https://doi.org/10.1007/s11626-020-00493-y
Fang Y, Huang W, Zhu X, Wang X, Wu X, Wang H, Hong W, Yan S, Zhang L, Deng Y, Wei W, Tu J, Zhu C (2024) Epigenetic regulatory axis MIR22-TET3-MTRNR2L2 represses fibroblast-like synoviocyte-mediated inflammation in rheumatoid arthritis. Arthritis Rheumatol 42795. https://doi.org/10.1002/art.42795
Sun Y, Zhang H, Qiu T, Liao L, Su X (2023) Epigenetic regulation of mesenchymal stem cell aging through histone modifications. Genes Dis 10(6):2443–2456. https://doi.org/10.1016/j.gendis.2022.10.030
Chen J, Yu H, Tan X, Mok SWF, Xie Y, Wang Y, Jiang X, Macrae VE, Lan L, Fu X, Zhu D (2023) PFKFB3-driven vascular smooth muscle cell glycolysis promotes vascular calcification via the altered FoxO3 and lactate production. FASEB J 37(10):e23182. https://doi.org/10.1096/fj.202300900R
Xu J, Ren Z, Niu T, Li S (2024) Epigenetic mechanism of miR-26b-5p-enriched MSCs-EVs attenuates spinal cord injury. Regen Ther 25:35–48. https://doi.org/10.1016/j.reth.2023.10.005
Varghese B, Babu S, Jala A, Das P, Raju R, Borkar RM, Adela R (2024) Integrative placental multi-omics analysis reveals perturbed pathways and potential prognostic biomarkers in gestational hypertension. Arch Med Res 55(1):102909. https://doi.org/10.1016/j.arcmed.2023.102909
Chadalawada S, Rathinam SR, Lalitha P, Kannan NB, Devarajan B (2023) Detection of microRNAs expression signatures in vitreous humor of intraocular tuberculosis. Mol Biol Rep 50(12):10061–10072. https://doi.org/10.1007/s11033-023-08819-1
Pedersen OB, Hvas A-M, Pasalic L, Kristensen SD, Grove EL, Nissen PH (2023) Platelet function and maturity and related microRNA expression in whole blood in patients with ST-segment elevation myocardial infarction. Thromb Haemost 124(3):192–202. https://doi.org/10.1055/s-0043-1776305
Li Y, Hu M, Xie J, Li S, Dai L (2023) Dysregulation of histone modifications in bone marrow mesenchymal stem cells during skeletal ageing: roles and therapeutic prospects. Stem Cell Res Ther 14(1):166. https://doi.org/10.1186/s13287-023-03393-6
Su P, Tian Y, Yang C, Ma X, Wang X, Pei J, Qian A (2018) Mesenchymal stem cell migration during bone formation and bone diseases therapy. Int J Mol Sci 19(8):2343. https://doi.org/10.3390/ijms19082343
Sonoyama W, Coppe C, Gronthos S, Shi S (2005) Skeletal stem cells in regenerative medicine. Curr Top Dev Biol 67:305–323. https://doi.org/10.1016/S0070-2153(05)67010-X
Wang F, Lian W, Lee MS, Weng W, Huang Y, Chen Y, Sun Y, Wu S, Chuang P, Ko J (2017) Histone demethylase UTX counteracts glucocorticoid deregulation of osteogenesis by modulating histone-dependent and -independent pathways. J Mol Med 95(5):499–512. https://doi.org/10.1007/s00109-017-1512-x
Qiu B, Yang E, Zheng Y, Zhang H (2023) Association between SPRY1 and TET3 in skin photoaging and natural aging mechanisms. J Cosmet Dermatol 23(4):1396–1403. https://doi.org/10.1111/jocd.16115
Salmerón-Bárcenas EG, Zacapala-Gómez AE, Torres-Rojas FI, Antonio-Véjar V, Ávila-López PA, Baños-Hernández CJ, Núñez-Martínez HN, Dircio-Maldonado R, Martínez-Carrillo DN, Ortiz-Ortiz J, Jiménez-Wences H (2024) TET enzymes and 5hmC levels in carcinogenesis and progression of breast cancer: potential therapeutic targets. Int J Mol Sci 25(1):272. https://doi.org/10.3390/ijms25010272
Liu Y, Wu J, Chen L, Zou J, Yang Q, Tian H, Zheng D, Ji Z, Cai J, Li Z, Chen Y (2024) ncRNAs-mediated overexpression of TET3 predicts unfavorable prognosis and correlates with immunotherapy efficacy in breast cancer. Heliyon 10(3):e24855. https://doi.org/10.1016/j.heliyon.2024.e24855
Katherine G, Walter AO, Emily W, Timothy JP, Gabriela F, Headtlove Essel D, Xiaoyang Z, Eric LS (2023) FoxA1/2-dependent epigenomic reprogramming drives lineage switching in lung adenocarcinoma. bioRxiv 10(30):564775. https://doi.org/10.1101/2023.10.30.564775
Wang C, Ju H, Zhou L, Zhu Y, Wu L, Deng X, Jiang L, Sun L, Xu Y (2024) TET3-mediated novel regulatory mechanism affecting trophoblast invasion and migration: implications for preeclampsia development. Placenta 147:31-41. https://doi.org/10.1016/j.placenta.2024.01.010
Dudakovic A, Jerez S, Deosthale PJ, Denbeigh JM, Paradise CR, Gluscevic M, Zan P, Begun DL, Camilleri ET, Pichurin O, Khani F, Thaler R, Lian JB, Stein GS, Westendorf JJ, Plotkin LI, van Wijnen AJ (2022) MicroRNA-101a enhances trabecular bone accrual in male mice. Sci Rep 12(1):13361. https://doi.org/10.1038/s41598-022-17579-0
Funding
We acknowledge the funding support from National Key Research and Development Program (Grant No. 2022YFA1104401 and 2022YFC2504201 to Z.F.), National Natural Science Foundation of China (Grant No. 82130028 to Z.F.), CAMS Innovation Fund for Medical Sciences (Grant No. 2019-I2M-5-031 to Z.F.), Innovation Research Team Project of Beijing Stomatological Hospital, Capital Medical University (Grant No. CXTD202204 to Z.F.) and Young Scientist Program of Beijing Stomatological Hospital, Capital Medical University (Grant No. YSP202113 to C.Z.).
Author information
Authors and Affiliations
Contributions
W.Y. wrote the first draft of the manuscript. W.Y. and C.Z. performed the material preparation, data collection and analysis. C.Z. and Z.F. contributed to the study conception and design. All authors reviewed the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
The animal study was approved by the Ethics Committee Agreement of Beijing Statistical Hospital (No. KQYY-202311-006).
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ye, W., Zhang, C. & Fan, Z. MiR-26b-5p/TET3 regulates the osteogenic differentiation of human bone mesenchymal stem cells and bone reconstruction in female rats with calvarial defects. Mol Biol Rep 51, 632 (2024). https://doi.org/10.1007/s11033-024-09577-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11033-024-09577-4