Abstract
Background
The involvement of malfunctioning glutamate systems in various central nervous system (CNS) disorders is widely acknowledged. Urolithin B, known for its neuroprotective and antioxidant properties, has shown potential as a therapeutic agent for these disorders. However, little is known about its protective effects against glutamate-induced toxicity in PC12 cells. Therefore, in this study, for the first time we aimed to investigate the ability of Urolithin B to reduce the cytotoxic effects of glutamate on PC12 cells.
Methods
Different non-toxic concentrations of urolithin B were applied to PC12 cells for 24 h before exposure to glutamate (10 mM). The cells were then analyzed for cell viability, intracellular reactive oxygen species (ROS), cell cycle arrest, apoptosis, and the expression of Bax and Bcl-2 genes.
Results
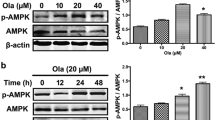
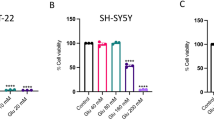
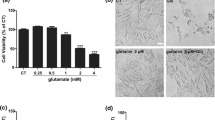
The results of MTT assay showed that glutamate at a concentration of 10 mM and urolithin B at a concentration of 114 μM can reduce PC12 cell viability by 50%. However, urolithin B at non-toxic concentrations of 4 and 8 μM significantly reduced glutamate-induced cytotoxicity (p < 0.01). Interestingly, treatment with glutamate significantly enhanced the intracellular ROS levels and apoptosis rate in PC12 cells, while pre-treatment with non-toxic concentrations of urolithin B significantly reduced these cytotoxic effects. The results also showed that pre-treatment with urolithin B can decrease the Bax (p < 0.05) and increase the Bcl-2 (p < 0.01) gene expression, which was dysregulated by glutamate.
Conclusions
Taken together, urolithin B may play a protective role through reducing oxidative stress and apoptosis against glutamate-induced toxicity in PC12 cells, which merits further investigations.





Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Abbreviations
- DMSO:
-
Dimethyl sulfoxide
- qRT-PCR:
-
Quantitative real-time polymerase chain reaction
- ROS:
-
Reactive oxygen species
- CNS:
-
Central nervous system
- MTT:
-
3-(4,5-Dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazolium bromide
- PI:
-
Propidium iodide
- FBS:
-
Fetal bovine serum
- TBHP:
-
Tert-butyl hydroperoxide
- PD:
-
Parkinson’s disease
- NO:
-
Nitric oxide
- IL-6:
-
Interleukin-6
- TNF-α:
-
Tumor necrosis factor-alpha
- PGE2:
-
Prostaglandin E2
References
Coffey CE et al (1998) Sex differences in brain aging: a quantitative magnetic resonance imaging study. Arch Neurol 55(2):169–179
Jin R et al (2002) Mechanism of activation and selectivity in a ligand-gated ion channel: structural and functional studies of GluR2 and quisqualate. Biochemistry 41(52):15635–15643
Molnar E, Isaac JT (2002) Developmental and activity dependent regulation of ionotropic glutamate receptors at synapses. ScientificWorldJournal 2:27–47
van Os S et al (2006) Excitatory amino acid release and electrocortical brain activity after hypoxemia in near-term lambs. Brain Dev 28(6):380–388
Camins A, Pallas M, Silvestre JS (2008) Apoptotic mechanisms involved in neurodegenerative diseases: experimental and therapeutic approaches. Methods Find Exp Clin Pharmacol 30(1):43–65
Benveniste H (2009) Glutamate, microdialysis, and cerebral ischemia: lost in translation? Anesthesiology 110(2):422–425
Monaghan DT, Bridges RJ, Cotman CW (1989) The excitatory amino acid receptors: their classes, pharmacology, and distinct properties in the function of the central nervous system. Annu Rev Pharmacol Toxicol 29:365–402
Bleich S et al (2003) Glutamate and the glutamate receptor system: a target for drug action. Int J Geriatr Psychiatry 18(Suppl 1):S33-40
Choi DW (1988) Glutamate neurotoxicity and diseases of the nervous system. Neuron 1(8):623–634
Murphy TH et al (1989) Glutamate toxicity in a neuronal cell line involves inhibition of cystine transport leading to oxidative stress. Neuron 2(6):1547–1558
Zabłocka A, Janusz M (2008) The two faces of reactive oxygen species. Postepy Hig Med Dosw (Online) 62:118–124
Tan S, Schubert D, Maher P (2001) Oxytosis: a novel form of programmed cell death. Curr Top Med Chem 1(6):497–506
Cerdá B et al (2003) Evaluation of the bioavailability and metabolism in the rat of punicalagin, an antioxidant polyphenol from pomegranate juice. Eur J Nutr 42(1):18–28
García-Villalba R et al (2019) Identification of novel urolithin metabolites in human feces and urine after the intake of a pomegranate extract. J Agric Food Chem 67(40):11099–11107
Espín JC et al (2013) Biological significance of urolithins, the gut microbial ellagic acid-derived metabolites: the evidence so far. Evid Based Complement Alternat Med 2013:270418
Seeram NP et al (2006) Pomegranate juice ellagitannin metabolites are present in human plasma and some persist in urine for up to 48 hours. J Nutr 136(10):2481–2485
Olennikov DN, Kashchenko NI, Chirikova NK (2015) In vitro bioaccessibility, human gut microbiota metabolites and hepatoprotective potential of chebulic ellagitannins: a case of Padma Hepaten® formulation. Nutrients 7(10):8456–8477
Abbasinezhad-Moud F et al (2023) The effects of urolithin B and auraptene on quinolinic acid-induced toxicity in the SH-SY5Y neuroblastoma cell line. Altern Lab Anim 51(1):30–38
Wiatrak B et al (2020) PC12 cell line: cell types, coating of culture vessels, differentiation and other culture conditions. Cells 9(4):958
Xie D et al (2022) The cellular model for Alzheimer’s disease research: PC12 cells. Front Mol Neurosci 15:1016559
Kinarivala N et al (2017) Passage variation of PC12 cells results in inconsistent susceptibility to externally induced apoptosis. ACS Chem Neurosci 8(1):82–88
Aranda A et al (2013) Dichloro-dihydro-fluorescein diacetate (DCFH-DA) assay: a quantitative method for oxidative stress assessment of nanoparticle-treated cells. Toxicol In Vitro 27(2):954–963
Ebrahimi S et al (2023) The in vitro anti-cancer synergy of neurokinin-1 receptor antagonist, aprepitant, and 5-aminolevulinic acid in glioblastoma. BioFactors 49(4):900–911
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods 25(4):402–408
Balestrino R, Schapira AHV (2020) Parkinson disease. Eur J Neurol 27(1):27–42
Tysnes OB, Storstein A (2017) Epidemiology of Parkinson’s disease. J Neural Transm (Vienna) 124(8):901–905
Chen P et al (2022) Recent advances and perspectives on the health benefits of urolithin B, a bioactive natural product derived from ellagitannins. Front Pharmacol 13:917266
Djedjibegovic J et al (2020) Ellagic acid-derived urolithins as modulators of oxidative stress. Oxid Med Cell Longev 2020:5194508
Moujalled D, Strasser A, Liddell JR (2021) Molecular mechanisms of cell death in neurological diseases. Cell Death Differ 28(7):2029–2044
Venderova K, Park DS (2012) Programmed cell death in Parkinson’s disease. Cold Spring Harb Perspect Med 2(8):a009365
Eidizade F et al (2023) Inhibition of glioblastoma proliferation, invasion, and migration by urolithin B through inducing G0/G1 arrest and targeting MMP-2/-9 expression and activity. BioFactors 49(2):379–389
Rahimi-Kalateh Shah Mohammad G et al (2023) Urolithin B loaded in cerium oxide nanoparticles enhances the anti-glioblastoma effects of free urolithin B in vitro. J Trace Elem Med Biol 78:127186
Lv MY et al (2019) Urolithin B suppresses tumor growth in hepatocellular carcinoma through inducing the inactivation of Wnt/β-catenin signaling. J Cell Biochem 120(10):17273–17282
Sánchez-González C et al (2014) Walnut polyphenol metabolites, urolithins A and B, inhibit the expression of the prostate-specific antigen and the androgen receptor in prostate cancer cells. Food Funct 5(11):2922–2930
Percário S et al (2020) Oxidative stress in Parkinson’s disease: potential benefits of antioxidant supplementation. Oxid Med Cell Longev 2020:2360872
DaSilva NA et al (2019) Pomegranate ellagitannin-gut microbial-derived metabolites, urolithins, inhibit neuroinflammation in vitro. Nutr Neurosci 22(3):185–195
Lee G et al (2019) Anti-inflammatory and antioxidant mechanisms of urolithin B in activated microglia. Phytomedicine 55:50–57
Qiu Z et al (2013) In vitro antioxidant and antiproliferative effects of ellagic acid and its colonic metabolite, urolithins, on human bladder cancer T24 cells. Food Chem Toxicol 59:428–437
Funding
This work’s authors recognize the following funding sources for their research, writing, and publication: Financial support for this project came from the Mashhad University of Medical Sciences (Grant No. 4011832).
Author information
Authors and Affiliations
Contributions
IA, MR, and AA contributed to the manuscript’s conception, design, acquisition, and drafting. FM, MKK, AH, and EG contributed to the interpretation and critically revised the manuscript. MS made distinctive contributions to the idea and demonstrated their specialized expertise to enhance the overall quality of the manuscript. All authors have thoroughly reviewed and provided their consent to the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
There are no known possible conflicts of interest.
Ethics approval and informed consent
The ethics committee of Mashhad University of Medical Sciences, Mashhad, Iran, approved the ethical issues of this study. It was a cell-based study, with no human sample included.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Aljabouri, I., Rostami, M., Mirzavi, F. et al. Urolithin B protects PC12 cells against glutamate-induced toxicity. Mol Biol Rep 51, 360 (2024). https://doi.org/10.1007/s11033-024-09236-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11033-024-09236-8