Abstract
Background
Pollution with heavy metals (HMs) is time- and concentration-dependent. Lead and zinc pollute the aquatic environment, causing severe health issues in aquatic animals.
Materials and methods
Nile tilapia, the predominant cultured fish in Egypt, were experimentally exposed to 10% of LC50 of lead nitrate (PbNO3) and zinc sulfate (ZnSO4). Samples were collected in three different periods, 4, 6, and 8 weeks, in addition to a trial to treat the experimental fish infected with Aeromonas hydrophila, with an antibiotic (florfenicol).
Results
Liver enzymes were linearly upsurged in a time-dependent manner in response to HMs exposure. ALT was 92.1 IU/l and AST was 82.53 IU/l after eight weeks. In the eighth week of the HMs exposure, in the hepatic tissue, the levels of glutathione peroxidase (GPx), catalase (CAT), and metallothionein (MT) were increased to 117.8 U/mg prot, 72.2 U/mg prot, and 154.5 U/mg prot, respectively. On exposure to HMs, gene expressions of some cytokines were linearly downregulated in a time-dependent manner compared to the control. After four weeks of exposure to the HMs, the oxidative burst activity (OBA) of immune cells was decreased compared to the control 9.33 and 10.3 cells, respectively. Meanwhile, the serum bactericidal activity (SBA) significantly declined to 18.5% compared to the control 32.6% after eight weeks of exposure. Clinical signs of A. hydrophila infection were exaggerated in polluted fish, with a mortality rate (MR) of 100%. The re-isolation rate of A. hydrophila was decreased in fish treated with florfenicol regardless of the pollution impacts after eight weeks of HMs exposure.
Conclusion
It could be concluded that the immune suppression and oxidative stress resulting from exposure to HMs are time-dependent. Clinical signs and post-mortem lesions in polluted fish infected with A. hydrophila were prominent. Infected-Nile tilapia had weak responses to florfenicol treatment due to HMs exposure.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In recent decades, Nile tilapia (Oreochromis niloticus) has been the most cultured and distributed worldwide, with net production exceeding 4.5 million tons in 2020 [1]. Although Nile tilapia originated from Africa and belongs to the Cichlidae family, it is cultivated worldwide due to its high growth rate, enhanced good feed utilization, economical maintenance, and high market demand. The optimum temperature range is 25 ± 3 °C for the ideal growth performance of Nile tilapia. Also, it can tolerate a wide temperature range of 16 to 38 °C out of this range, it stops the feed intake, and mortality could be expected [1].
Environmental pollution has attracted global attention in the last decades and is becoming a severe and vital challenge for human society [1, 2]. Heavy metals released in the aquatic environment through municipal discharges wastes of agricultural industrial activity, mining, combustion of fossil fuels, and wastewater treatment plants [3], resulting in environmental pollution and raising the risk of bioaccumulation in fish tissues, threatening the life of aquatic animal, also feeding on aquatic products impacts the human health [4]. In Egypt, water polluted with heavy metals became a public health issue as domestic, agricultural, and industrial is discharged into the aquatic environment, the nature of heavy metals is toxic and could accumulate in the flesh of the aquatic animals, in turn transferred to the human body [5,6,7,8,9]. Heavy metals accumulate in fish organs, inducing oxidative stress [10]. The alterations occurred in antioxidant enzymes values considered oxidative stress biomarkers in fish that were exposed to environmental pollutants [11, 12]. Also, the disturbances of gene expression of metallothionein (MT) and heat shock proteins (Hsps) could be used as biomarkers for environmental pollutants [13, 14].
Aquatic animals that reared in the lead (Pb) polluted areas showed deleterious biochemical and physiological status [15], such as growth inhibition [16, 17], downregulating of hematological indices, initiating inflammation and apoptosis, and oxidative stress injury [18, 19]. These impacts resulted in immune depression via disturbing cytokine gene expressions [17, 18].
Zinc, a heavy metal, is widely distributed in the aquatic environment [20], it could persist for long periods in the environment and could not be biodegraded [21], causing an oxidative status and disturbing acid-base balance, damaging the gills of the aquatic animals [22]. Fish exposed to high concentrations of Zn causing structural damages adversely affect growth, development, and survival [23], accumulating in gills and leading to death by hypoxia [24, 25].
Fish exposed to sublethal concentrations of Zn showed low hatchability and survival rates, along with decreased hematological parameters and adverse alteration behaviors [26, 27]. In Egypt, Zn is commonly present in ecosystems in which Nile tilapia could be reared (fish farms) and caught (open water; Lakes, water canals, and Nile River), the concentration ranged between 0.004 and 0.46 mg/L [28], whereas Abdel-Baky et al. [29] found Zn concentration of 7.94 mg/L.
This work gives insight into treating of bacterial infection in Nile tilapia during exposure to a mixture of lead nitrate (PbNO3) and zinc sulfate (ZnSO4) pollution.
Materials and methods
Investigation sites
Three hundred Nile tilapia were purchased from a private freshwater fish farm at village Tolompate 7 in Kafrelsheikh governorate, Egypt. The collected fish were tranquilized in the fish farm using MS-222 (SyncaineR, Syndel, Canada) at a dose of 40 mg/L, and then the fish were transferred live to the wet laboratory of the Animal Health Research Institute at Kafrelsheikh. The acclimatization process was performed according to the recommendations [30,31,32]. On arrival, fish were subjected to an iodine bath (BetadineR active ingredient povidone-iodine, 5%, Nile Company for Pharmaceuticals, Egypt) at a dose of 20 ppm/L before stocking in a fiberglass tank 1.5 × 1.5 × 1 m for fourteen days, at the end of the Acclimatization period fish restored regular feeding behavioral. Two hundred and forty healthy fish were used in the experimental investigation. Water samples were collected along with the collected fish to detect the levels of Pb and Zn. At the end of the experimental period, fish were euthanized using MS-222 at a dose of 250 mg/L, and fish were kept for ten minutes after ceasing the operculum movements [33].
Experimental trial
In experimental exposure in the lab, Two hundred and forty Nile tilapia (30 ± 5 g b.w.) were distributed into glass aquaria containing 10% LC50 of lead nitrate (PbNO3) [34] and zinc sulfate (ZnSO4) [35], 14.33 mg/L and 6.398 mg/L, respectively, for 4, 6, and 8 weeks.
Bacterial infection
At the 4, 6, and 8 weeks of stocking in polluted water, Nile tilapia were experimentally infected with Aeromonas hydrophila 0.3 × 104 CFU, which equals 10% of bacterial LD50. Ten Nile tilapia from each group were randomly chosen at 4, 6, and weeks of the HMs exposure and injected via intraperitoneal (IP) route with LD50 (2.4 × 105 CFU) of an A. hydrophila AHRAS2 pathogenic strain (accession number MW092007) which was isolated from mass mortality of Nile tilapia reared in fish cages, bacteria were identified by Sherif and Abuleila [36]. In addition, ten fish from the control group were injected with pure saline solution (0.65%) and were considered negative controls [37]. The infected Nile tilapia were observed for two weeks for deaths. The mortality rate (MR) was estimated as follows:
MR (%) = (number of fish deaths in a specific period ∕ total fish population during that period) × 100.
Experimental Nile tilapia challenged with A. hydrophila and subjected to trials of re-isolation according to the following equation:
Re-isolated (%) = (number of challenged fish harbored injected microbe in a specific period ∕ total fish population during that period) × 100.
Examination of farm and experimental fish
Following the recommendations of Amlacher [38], the experimental Nile tilapia were clinically examined for abnormal signs such as exophthalmia, dentated fines, skin hemorrhages, and detached scales, in addition to examination of the internal organs for changes in color, size, hemorrhages.
Heavy metals levels
The concentrations of lead (Pb) and zinc (Zn) in the experimental fish were detected in different body tissues. The collected fish were washed with distilled water, put in clean plastic bags, and stored frozen until analysis was carried out according to procedures recommended by A.O.A.C. [39]. Heavy metal analysis was carried out for water, sediment, and fish tissues according to APHA [40] using an atomic absorption spectrophotometer (Thermo electron corporations series AA Spectrometer).
Liver enzymes and Metallothionein
Liver enzymes, the experimental fish blood was collected from the tail vein and centrifuged to obtain sera, which were used to colorimetrically determine aspartate amino transaminase (AST) and alanine amino transaminase (ALT) using a spectrophotometer according to Reitman and Frankel [41].
Metallothionein (MT) content in hepatic tissues of the experimental Nile tilapia was spectrophotometrically measured following the method described by Derango and Page [42].
Cytokines gene expression using quantitative RT-PCR
The impact of water pollution on the expression of immune-related genes was assessed using RT-PCR. From the hepatic tissues, the RNA was extracted with Trizol reagent (iNtRON Biotechnology Inc., Korea), and samples were collected from three Nile tilapia at 0, 4, 6, and 8 weeks of exposure to PbNO3 and ZnSO4 and by using Nanodrop D-1000 spectrophotometer (NanoDrop Technologies Inc., USA) the obtained RNA was evaluated for its quality and quantity the kept at − 80 °C.
The complementary DNA (cDNA) was formed with SensiFAST cDNA kits (Bioline, USA) for interleukin (IL)-1β, tumor necrosis factor (TNF)-β, transforming growth factor (TGF)-β2, insulin-like growth factor (IGF)1, C-type lysozyme, and heat shock protein (Hsp)70. Nile tilapia-specific primers are presented in Table 1; the β-actin gene was the housekeeping gene. The data of gene expressions from RT-PCR were assessed using Eq. 2−ΔΔCT [43].
Antioxidants enzymes activity
Glutathione peroxidase (GPx) (EC 1.11.1.9) activity in the hepatic tissue of the experimental Nile tilapia was calculated using Mohandas et al. [44] method. The ingredients of the reaction mixture were 1.44 ml of 0.05 M PBS (pH 7.0), 0.1 ml of 1 mM EDTA, 0.1 mM sodium azide, 0.05 ml of glutathione reductase (GR; 1U/ml), 0.1 ml of 1 mM glutathione (GSH), 0.1 ml of 2 mM NADPH, 0.01 ml of 0.25 mM H2O2 and 0.1 ml of 10% PMS in a total volume of 2 ml. The disappearance of NADPH was spectrophotometrically recorded at 340 nm. Enzyme activity was calculated as nmol NADP reduced/min/mg protein using a molar extinction coefficient of 6.22 × 103/M/cm.
Catalase CAT (EC 1.11.1.6) activity in hepatic tissue of the experimental Nile tilapia was measured spectrophotometrically at 240 nm and measured as µmol H2O2 decomposed/ mg protein/min according to a method described by Lartillot et al. [45].
Immune assay
-
(a)
Serum bactericidal activity (SBA).
The SBA of the experimental Nile tilapia was calculated following the methods developed by Kajita et al. [46]. Briefly, equal volumes of A. hydrophila bacterial suspension containing 1 × 106 CFU/mL and experimental fish serum of about 100 µL were mixed parallel with the blank in which sterile phosphate-buffered saline (PBS) was used instead of fish serum. The ten-fold serial dilutions were made and incubated at 37 °C/24 h, and then bacterial colonies were counted on nutrient agar plates.
-
(b)
Neutrophil glass-adhesion.
Oxidative burst activity (OBA) of the experimental Nile tilapia was calculated following the method of Jang et al. [47], using nitro blue tetrazolium (NBT) test. In 96-well, leukocyte suspension (50 µL containing 50 cells) previously prepared from the blood of the experimental fish was loaded and incubated at 30 °C/1 h, then wells were rinsed with PBS and put at room temperature for one hour. After that, 50 µL of NBT was added to each well and fixed by methanol (30%), then left for five minutes. The wells were dried by filling with 60 µL of 2 mM potassium hydroxide and 70 µL of dimethyl sulfoxide. A plate reader performed the measures at 540 nm.
Statistical examination of the obtained values
The pollution impacts on Nile tilapia health were statistically analyzed by the analysis of variance between different periods of exposure using one-way ANOVA and the significance of differences between means by using Duncan’s Multiple Range at a level of 0.05 [48].
The biosafety procedures used in the experiment
All dead fish and remaining fish after the end of the experiment were burned in the fixed incinerator in the laboratory. The biosafety measures followed the pathogen regulation directorate for infectious substances (A. hydrophila) [49].
Results
The concentration of heavy metals pb and zn in the experimental fish tissues
In Table 2, Nile tilapia were subjected to Pb and Zn pollution (PbNO3 and ZnSO4 at a concentration of 14.33 mg/L and 6.398 mg/L, respectively), and Pb and Zn concentrations were measured in muscles and liver tissues at various periods. The control fish had undetectable levels of HMs, as did those exposed for four weeks. Detectable levels of Pb and Zn were detected after six and eight weeks of exposure, respectively, and the levels were associated with the time factor. Regardless of time factor, the level of Pb in liver tissues was higher compared to muscle tissues 5.33 and 6.93; 2.2 and 3.81 µg/g dry w.t., respectively. Meanwhile, Zn levels had the same trend.
Liver enzymes and antioxidants and metallothionein
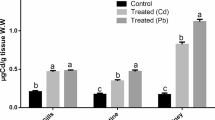
In Table 3, Nile tilapia exposed to a mixture of PbNO3 and ZnSO4 showed a significant deviation from normal physiological status. Liver enzymes ALT and AST showed significant and linear upsurge with time factor compared to the unpolluted group. The highest value was in the eighth week, 92.1 and 82.53 IU/l, respectively, which was about five times the control, 21.77 IU/l. On HMs exposure, antioxidants GPx (Fig. 1) and CAT (Fig. 2) had a significant rise of about 50% over the control after 4 and 6 weeks, 87.5 and 91.2; 44.1 and 53.77 U/mg prot, while their values were duplicated after eighth weeks. A ten-fold increase was observed in MT values (Fig. 3) compared to the control regardless of the time factor; the highest value was 154.5 U/mg prot after the eighth week.
Immunity examination
Gene expression of some immune related genes
In Fig. 4, overall gene expression of some immune-related genes (IL-1β, TNF-b, TGF-β2, IGF1, C-type lysozyme, and Hsp70) indicated that HMs could modulate the immune status of Nile tilapia. At the same time, the expressions of IL-1β, TNF-b, and Hsp70 were linearly declined with time. In contrast, C-type lysozyme increased with time and reached 1.14 fold change compared to the control 1 fold change. Whereas, TGF-β2 and IGF1 increased in the four week of exposure (1.17 and 1.14 fold change), then they were declined until (0.53 and 0.52 fold change) in the eighth week compared to the control (1 and 1 fold change), respectively.
Immune status tests
In Table 4, the activity of immune cells was examined via OBA test, Nile tilapia exposed to HMs for four weeks showed insignificant difference with the control 9.33 and 10.3 cells, whereas the activity significantly declined after 4 and 8 weeks of HMs exposure 83.3 and 5.33; 12.3 and 9.67 cells. No alterations were recorded in the SBA of Nile tilapia after 4 and 6 weeks of HMs exposure, whereas after the eighth week, a drastic decline was observed to 18.5% compared to the control 32.6%.
Mortality rate
In Fig. 5, Nile tilapia was experimentally infected with pathogenic A. hydrophila via intraperitoneal injection with LD50. The clinical signs were partially off-food, loss of body color, slight pop-eye, and hemorrhages on the skin, while post-mortem lesions were partially empty intestine and splenomegaly with different sizes. In Table 5, Nile tilapia unexposed to HMs pollution had MR ranged between 50% and 70% after experimental infection with LD50A. hydrophila, whereas for those exposed to HMs pollution, the MR was 100 after eight weeks of exposure.
In Table 6, after experimental infection with 10% of LD50 of A. hydrophila, MR% was higher in Nile tilapia exposed to HMs compared to the control 40% and 0 at the eighth week. Florfenicol treatment could decrease the MR to 10% in exposed Nile tilapia after six and eight weeks of HMs exposure. After exposure to HMs, the re-isolation of A. hydrophila was decreased in florfenicol fish compared to the untreated ones 40% and 50%; 100%, respectively.
In Fig. 6, Nile tilapia was experimentally infected with 10% LD50 of A. hydrophila. Clinical signs were slight hemorrhages on the skin, tail dentations, and slight pop-eye. Meanwhile, post-mortem lesions were slight splenomegaly and full-intestine.
Discussion
Environmental pollution affected fish health, which could be monitored by pathological changes in the hepatic tissue [50], for example, heavy metals toxicity, which resulted in elevated liver enzymes values such as ALT and AST [51, 52]. In this investigation, long-term exposure of Nile tilapia to polluted water (PbNO3 and ZnSO4 mixture) significantly resulted in severe physiological alterations such as elevated liver enzymes ALT and AST that were about five times as the control after eight weeks. In accordance, the liver enzymes ALT and AST are very sensitive to any pathological changes in the hepatic tissue, so they could be considered the main biomarkers of body tissue damage [53,54,55,56,57]. Similarly, Pb toxicity could damage hepatic tissue, increasing liver enzymes in fish serum Nile tilapia [58] and common carp [59]. Accordingly, Firat and Kargin [60] found that the level of AST and ALT were increased in the serum of Zn-exposed Nile tilapia, indicating hepatic dysfunction.
Heavy metals pollution induces the production of reactive oxygen species (ROS such as superoxide, hydroxyle, and peroxides) in animal tissues, causing oxidative damage and destroying cell walls and components [61, 62], which stimulates antioxidants enzymes SOD, GPx, and CAT to counteract the propagated superoxide radicals [63]. In this work, hepatic GPx and CAT rose about double the control in Nile tilapia exposed to PbNO3 and ZnSO4 mixture. Similarly, ROS were produced in response to heavy metals bioaccumulation in fish bodies, leading to stimulate SOD, which catalytically eliminates oxidative stress [51, 64] after eight weeks. In accordance, the alterations of antioxidant enzymes are considered bioindicators for heavy metals pollution [65]; in case antioxidant enzymes cannot scavenge the propagated ROS due to Pb toxicity, cell membranes will be damaged despite upregulation of the expression of genes related to antioxidant enzymes production [66, 67].
After eight weeks of PbNO3 and ZnSO4 exposure, the hepatic MT was significantly increased compared to the control, 154.5 and 16.03 U/mg. prot, respectively. In accordance, heavy metal pollution induces the production of hepatic MT, which could be used as a biomarker, MT protects hepatic cells from being damaged by propagated ROS, decreasing oxidative stress [68]. Similarly, Zn and Cd toxicity could induce higher expression gene of MT in common carp and zebrafish (Danio rerio) [69] gudgeons (Gobio gobio) from Cd-contaminated sites in Flanders, Belgium [70]. Similarly, Darwish et al. [71] stated that MT in mammalian and non-mammalian tissues could be considered a biomarker for environmental pollution with heavy metals such as Pb and Cd.
As signaling molecules, cytokines are mediators generated by the immune cells and are necessary for the host’s defensive system [72]. In the exposed Nile tilapia, the gene expressions of IL-1β, TNF-b, and Hsp70 were linearly declined in response to polluted water (PbNO3 and ZnSO4 mixture) in the hepatic tissues of Nile tilapia, indicating immunosuppression status. In accordance, alterations in the gene expressions of cytokines in the hematopoietic tissues were recorded after exposure to genotoxic compounds as HMs [73, 74]. In contrast, Hsps are not only induced by heat shock but also by contamination with HMs that trigger oxidative stress [70, 75]. In the experimental Nile tilapia, the expression of C-type lysozyme was linearly increased till the eighth week, whereas TGF-β2 and IGF1 genes were increased after four weeks of exposure and then linearly declined in the next four weeks. Accordingly, HMs pollution resulted in tissue injuries in the exposed fish [76, 77]. The decline could be due to DNA damage and immune system adaptation to long-period exposure.
In this study, there was a drastic decline in the activity of immune cells OBA and SBA of Nile tilapia reared in polluted water (PbNO3 and ZnSO4 mixture). Hence, the immune status is evaluated by assessing the serum bactericidal activity, which could disrupt bacterial cell walls, so it could be considered one of the typical methods to assess fish immunity [78]. Accordingly, ROS induces oxidative stress causing injuries and damage to the membrane of blood cells [11, 79]. Besides, fish serum possesses a bactericidal activity against Gram-positive and Gram-negative bacteria via stimulating the complement system to combat the infection [78, 80].
Our results indicated the poor health status of stressed Nile tilapia, which became vulnerable to bacterial infection [81,82,83]. Similarly, aquatic pollutants could impair the immune system of fish; low SBA and OBA were observed along with downregulation of IL-1β, IL-8, TNF-α, HSP70, and IL-10 gene expressions [84]. In accordance, blood cells play a crucial role in fish immunity due to their phagocytic function against pathogens such as bacteria, parasites, and viruses [85], oxidative stress results in cellular damage in fish [86]. In this work, after experimental infection with 10% of LD50 of A. hydrophila, MR was higher in Nile tilapia exposed to heavy metals 40% compared to the control. Accordingly, with heavy metals exposure, Nile tilapia was susceptible to Streptococcus agalactiae infection, and their survivability declined [84]. Similarly, in aquatic systems, heavy metals rapidly accumulate in fish tissues, hindering their normal physiological functions and leading to high mortalities [87, 88]. In addition, Abdel-Tawwab et al. [84] found that Nile tilapia could withstand exposure to water-born Zn regimes, showing high survival rates of 96.7% and 100.0%. This result could be due to low concentration and long periods of water-born Zn exposure compared to our results.
Conclusion
From the obtained results, it was clear that the immune-oxidative status was adversely impacted in Nile tilapia, which was exposed to lead and zinc pollution. In addition, the trial of antibiotic treatment failed to achieve its goal of eliminating the pathogenic A. hydrophila. In addition, the clinical signs and post-mortem lesions were amplified in polluted fish. Therefore, it is recommended that the HMs residues and immune-oxidative status should be evaluated before deciding on antibiotic treatment.
Data Availability
Data are available on request from the corresponding author.
Code Availability
Not applicable.
References
FAO (2020) Agriculture Organization of the United Nations the state of world fisheries and aquaculture 2020: sustainability in action Rome. Food and Agriculture Organization of the United Nations, pp 1–244
Ali H, Khan E, Ilahi I (2019) Environmental chemistry and ecotoxicology of hazardous heavy metals: environmental persistence, toxicity, and bioaccumulation. J chem 2019: 6730305. https://doi.org/10.1155/2019/6730305
Gheorghe S, Stoica C, Vasile GG, Nita-Lazar M, Stanescu E, Lucaciu IE (2017) Metals toxic effects in aquatic ecosystems: modulators of water quality. Water Qual 87:59–89
Briffa J, Sinagra E, Blundell R (2020) Heavy metal pollution in the environment and their toxicological effects on humans. Heliyon 6(9):e04691
George MS, Nwaichi EO, Monago-Ighorode C (2023) Heavy metals and Hydrocarbon Level in Nile Tilapia (Oreochromis niloticus), Mullet Fish (Liza Falcipinus) and crab (Callinectes amnicola) from Crude Oil Contaminated Jetty Rivers in Port Harcourt. Nigeria JASEM 27(4):883–891
Malhat F (2011) Distribution of heavy metal residues in fish from the River Nile tributaries in Egypt. Bull Environ Contam Toxicol 87:163–165
Sherif AH, Alsokary ET, Esam HA (2019) Assessment of titanium dioxide nanoparticle as treatment of Aeromonas hydrophila Infection in Oreochromis niloticus. J Hellenic Vet Med Soc 70(3):1697–1706. https://doi.org/10.12681/jhvms.21796
Sherif AH, Khalil RH, Tanekhy M, Sabry NM, Harfoush MA, Elnagar MA (2022) Lactobacillus plantarum ameliorates the immunological impacts of titanium dioxide nanoparticles (rutile) in Oreochromis niloticus. Aquac Res 53:3736–3747. https://doi.org/10.1111/are.15877
Sherif AH, Elkasef M, Mahfouz ME, Kasem EA (2023) Impacts of dietary zinc oxide nanoparticles on the growth and immunity of Nile tilapia could be ameliorated using Nigella sativa oil. J Trace Elem Med Biol 79:127265. https://doi.org/10.1016/j.jtemb.2023.127265
Prieto AI, Jos Á, Pichardo S, Moreno I, Cameán AM (2006) Differential oxidative stress responses to microcystins LR and RR in intraperitoneally exposed tilapia fish (Oreochromis Sp). Aquat Toxicol 77(3):314–321. https://doi.org/10.1016/j.aquatox.2005.12.012
Ahmad I, Pacheco M, Santos MA (2006) Anguilla anguilla L. oxidative stress biomarkers: an in situ study of freshwater wetland ecosystem (Pateira De Fermentelos, Portugal). Chemosphere 65(6):952–962
Tom M, Chen N, Segev M, Herut B, Rinkevich B (2004) Quantifying fish metallothionein transcript by real time PCR for its utilization as an environmental biomarker. Mar Pollut Bull 48(7–8):705–710
Piano A, Valbonesi P, Fabbri E (2004) Expression of cytoprotective proteins, heat shock protein 70and metallothioneins, in tissues ofOstrea edulis exposed to heat andheavy metals. Cell Stress Chaperon 9(2):134–142
Sturve J, Berglund Å, Balk L et al (2005) Effects of dredging in Göteborg Harbor, Sweden, assessed by biomarkers in eelpout (Zoarces viviparus). Environ Toxicol Chemistry: Int J 24(8):1951–1961
Alam M, Rohani MF, Hossain MS (2023) Heavy metals accumulation in some important fish species cultured in commercial fish farm of Natore, Bangladesh and possible health risk evaluation. Emerg Contam 9(4):100254
Zhai Q, Wang H, Tian F, Zhao J, Zhang H, Chen W (2017) Dietary Lactobacillus plantarum supplementation decreases tissue lead accumulation and alleviates lead toxicity in Nile tilapia (Oreochromis niloticus). Aquac Res 48(9):5094–5103
Giri SS, Kim MJ, Kim SG et al (2021) Role of dietary curcumin against waterborne lead toxicity in common carp Cyprinus carpio. Ecotoxicol Environ Saf 219:112318
Lee JW, Choi H, Hwang UK et al (2019) Toxic effects of lead exposure on bioaccumulation, oxidative stress, neurotoxicity, and immune responses in fish: a review. Environ Toxicol Pharmacol 68:101–108
Kiran Kumar E, Midhun SJ, Vysakh A, James TJ (2021) Antagonistic effects of dietary Moringa oleifera on hemato-biochemical and oxidative stress of lead nitrate intoxicated Nile tilapia, Oreochromis niloticus. Aquac Res 52(12):6164–6178
Bengeri KV, Patil HS (1986) Respiration, liver glycogen and bioaccumulation in Labeo rohita exposed to zinc. Ind J Comp Anim Physiol 4:79–84
Everall NC, MacFarlane NAA, Sedgwick RW (1989) The interactions of water hardness and pH with the acute toxicity of zinc to the brown trout, Salmo trutta L. J Fish Biol 35(1):27–36
Hogstrand CH, Wilson RW, Polgar DO, Wood CM (1994) Effects of zinc on the kinetics of branchial calcium uptake in freshwater rainbow trout during adaptation to waterborne zinc. J Exp Biol 186(1):55–73
Tuurala H, Soivio A (1982) Structural and circulatory changes in the secondary lamellae of Salmo Gairdneri gills after sublethal exposures to dehydroabietic acid and zinc. Aquat Toxicol 2(1):21–29
Hughes GM, Tort L (1985) Cardio-respiratory responses of rainbow trout during recovery from zinc treatment. Environ Pollut A. Ecol Biol 37(3):255–266. https://doi.org/10.1016/0143-1471(85)90045-5
Crespso S, Flos R, Balasch J, Alonso G (1979) Zinc in the gills of dogfish (Scychorhinus canicula) related to experimental aquatic zinc pollution. Comp Biochem Physiol 63:261–266
Cardeihac PT, Simpson CF, White FH, Thompson NP, Carr WE (1981) Evidence of metal Poisoning in acute death of large red dram Scranops Ocellata. Bull Environ Contam Toxicol 27(5):639–644
Annune PA, Iyaniwura TT, Ebele S, Oladimeji AA (1994) Effects of sublethal concentrations of zinc on haematological parameters of freshwater fishes, Clarias gariepinus(Burch) and Oreochromis niloticus(Trewavas). J Aquat Sci 9:1–6
Saeed SM, Shaker IM (2008) Assessment of heavy metals pollution in water and sediments and their effect on Oreochromis niloticus in the northern delta lakes, Egypt. Proceedings of the 8th International Symposium on Tilapia in Aquaculture, H. Elghoubashy, K. Fitzsimmons, and A.S. Diab (Editors), Cairo International Convention Center, Cairo, Egypt, 12–14 October 2008, pp 475–490
Abdel-Baky TE, Hagras AE, Hassan SH, Zyadah MA (1998) Environmental impact assessment of pollution in Lake Manzala, I-Distribution of some heavy metals in water and sediment. J Egypt Ger Soc Zool 26:25–38
Eldessouki EA, Salama SSA, Mohamed R, Sherif AH (2023) Using Nutraceutical to alleviate transportation stress in the Nile tilapia. Egypt J Aquat Biol Fish 27(1):413–429. https://doi.org/10.21608/ejabf.2023.287741
Sherif AH, Eldessouki EA, Sabry NM, Ali NG (2023) The protective role of iodine and MS-222 against stress response and bacterial Infections during Nile tilapia (Oreochromis niloticus) transportation. Aquac Int 31(1):401–4163. https://doi.org/10.1007/s10499-022-00984-7
Sherif AH, Farag EA, Mahmoud AE (2023) Temperature fluctuation alters immuno-antioxidant response and enhances the susceptibility of Oreochromis niloticus to Aeromonas hydrophila challenge. Aquac Int 1–14. https://doi.org/10.1007/s10499-023-01263-9
Noga EJ (2010) Pharmacopoeia. Fish Disease: diagnosis and treatment, 2nd edn. John Wiley & Sons, pp 375–420
Sherif AH, Al-Sokary ET, Rizk WF, Mahfouz ME (2020) Immune status of Oreochromis niloticus subjected to long-term lead nitrate exposure and a Arthrospira platensis treatment trial. Environ Toxicol Pharmacol 76:103352. https://doi.org/10.1016/j.etap.2020.103352
Abdel-Tawwab M, El-Sayed GO, Shady SHH (2011) Acute toxicity of water-born zinc in Nile tilapia, Oreochromis niloticus (L.) fingerlings. In Proceedings of the Ninth International Symposium on Tilapia in Aquaculture, Shanghai Ocean Univ. China (pp. 44–50)
Sherif AH, AbuLeila RH (2022) Prevalence of some pathogenic bacteria in caged- Nile Tilapia (Oreochromis Niloticus) and their possible treatment. Jordan J Biol Sci 15(2):239–247. https://doi.org/10.54319/jjbs/150211
Boijink CDL, Brandão DA, Vargas ACD, Costa MMD, Renosto AV (2001) Inoculação De suspensão Bacteriana De Plesiomonas shigelloides em jundiá, Rhamdia quelen (teleostei: pimelodidae). Ciênc Rural 31:497–501
Amlacher E (1970) Text book of fish Disease. T.E.S publication, New Jersey, USA, pp 117–135
AOAC, Association of Official Agriculture Chemists Official (1990) Methods of analysis 15th Ed. Published by the A.O.A.C. Benjamin Franklin Station. Wash D C 1(1):41–44
APHA, American Public Health Association (1992) Standard method for the examination of water and waste water 20th ed. Washington, D.C
Reitman S, Frankel S (1957) Determination of AST and ALT in serum. Am J Clin Pathol 28:56–68
Derango R, Page J (1996) The quantitation of coupled bead antibody by enzyme-linked immunosorbent assay. J Immunoass Immunochem 17(2):145–153
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2 – ∆∆CT method. methods 25(4): 402–408
Mohandas J, Marshall JJ, Duggin GG, Horvath JS, Tiller DJ (1984) Differential distribution of glutathione and glutathione-related enzymes in rabbit kidney: possible implications in analgesic Nephropathy. Biochem Pharmacol 33(11):1801–1807
Lartillot S, Kedziora P, Athias A (1988) Purification and characterization of a new fungal catalase. Prep Biochem 18(3):241–246. https://doi.org/10.1080/00327488808062526
Kajita Y, Sakai M, Atsuta S, Kobayashi M (1990) The immunomodulatory effects of levamisole on rainbow trout, Oncorhynchus mykiss. Fish Pathol 25(2):93–98
Jang SI, Hardie LJ, Secombes CJ (1995) Elevation of rainbow trout Oncorhynchus mykiss macrophage respiratory burst activity with macrophage-derived supernatants. J Leukoc Biol 57(6):943–947. https://doi.org/10.1002/jlb.57.6.943
SPSS (2004) Statistical and package for social science, SPSS for windows release 14.0.0, 19 June, 2004 (pp. 1989–2004). Standard Version. Copyright SPSS Inc
Public Health Agency of Canada (2010) The honourable Leona Aglukkaq. P.C., M.P. Minister of Health
Ansaldo M, Luquet CM, Evelson PA, Polo JM, Llesuy S (2000) Antioxidant levels from different Antarctic fish caught around South Georgia Island and Shag Rocks. Polar Biol 23:160–165
Farombi EO, Adelowo OA, Ajimoko YR (2007) Biomarkers of oxidative stress and heavy metal levels as indicators of environmental pollution in African cat fish (Clarias gariepinus) from Nigeria Ogun River. Int J Environ Res 4(2):158–165
Pandey S, Parvez S, Ansari RA et al (2008) Effects of exposure to multiple trace metals on biochemical, histological and ultrastructural features of gills of a freshwater fish, Channa punctata Bloch. Chem Biol Interact 174(3):183–192
Javed M (2012) Tissue-specific bio-accumulation of metals in fish during chronic waterborne and dietary exposures. Pak Vet J 32:567–570
Metwally AE, Ibrahim D, Khater SI (2015) Effects of supplementing broiler diets with CreAMINO® on broiler performance, carcass traits and the expression of muscle growth related genes. ROAVS 5(11):435–442
Ibrahim D, El Sayed R, Abdelfattah-Hassan A, Morshedy AM (2019) Creatine or guanidinoacetic acid? Which is more effective at enhancing growth, tissue creatine stores, quality of meat, and genes controlling growth/myogenesis in Mulard ducks. J Appl Anim Res 47(1):159–166
Sherif AH, Gouda MY, Zommara MA, Abd El-Rahim AH, Mahrous KF, Salama ASS (2021) Inhibitory effect of nano selenium on the recurrence of Aeromonas hydrophila bacteria in Cyprinus carpio. Egypt J Aquat Biol Fish 3713–738. https://doi.org/10.21608/EJABF.2021.180901
Sherif AH, Elshenawy AM, Attia AA, Salama SAA (2021) Effect of aflatoxin B1 on farmed Cyprinus carpio in conjunction with bacterial Infection. Egypt J Aquat Biol Fish 25(2):465–485. https://doi.org/10.21608/EJABF.2021.164686
Rahman MS, Molla AH, Saha N, Rahman A (2012) Study on heavy metals levels and its risk assessment in some edible fishes from Bangshi River, Savar, Dhaka, Bangladesh. Food chem 134(4):1847–1854
Rajamanickam V, Muthuswamy N (2008) Effect of heavy metals induced toxicity on metabolic biomarkers in common carp (Cyprinus carpio L). Maejo Int J Sci Technol 2(1):192–200
Fırat Ö, Kargin F (2010) Individual and combined effects of heavy metals on serum biochemistry of Nile tilapia Oreochromis Niloticus. Arch Environ Contam Toxicol 58:151–157
Burgos-Aceves MA, Abo-Al-Ela HG, Faggio C (2021) Physiological and metabolic approach of plastic additive effects: Immune cells responses. J Hazard Mater 404:124114
Gobi N, Vaseeharan B, Rekha R, Vijayakumar S, Faggio C (2018) Bioaccumulation, cytotoxicity and oxidative stress of the acute exposure selenium in Oreochromis mossambicus. Ecotoxicol Environ Saf 162:147–159
Krajcovicova-Kudlackova M, Ursinyova M, Blazicek P, Spustova V, Ginter E, Hladikova V, Klvanova J (2003) Free radical Disease prevention and nutrition. Bratisl Lek Listy 104(2):64–68
Kádár E, Costa V, Santos RS (2006) Distribution of micro-essential (Fe, Cu, Zn) and toxic (hg) metals in tissues of two nutritionally distinct hydrothermal shrimps. Sci Total Environ 358(1–3):143–150
Jin Y, Zhang X, Shu L et al (2010) Oxidative stress response and gene expression with atrazine exposure in adult female zebrafish (Danio rerio). Chemosphere 78(7):846–852
Abd El-Hamid MI, Ibrahim SM, Eldemery F et al (2021) Dietary cinnamaldehyde nanoemulsion boosts growth and transcriptomes of antioxidant and immune related genes to fight Streptococcus agalactiae Infection in Nile tilapia (Oreochromis niloticus). Fish Shellfish Immunol 113:96–105
Ni H, Peng L, Gao X, Ji H, Ma J, Li Y, Jiang S (2019) Effects of maduramicin on adult zebrafish (Danio rerio): acute toxicity, tissue damage and oxidative stress. Ecotoxicol Environ Saf 168:249–259
Pathiratne A, Chandrasekera LWHU, Pathiratne KAS (2009) Use of biomarkers in Nile tilapia (Oreochromis niloticus) to assess the impacts of pollution in Bolgoda Lake, an urban water body in Sri Lanka. Environ Monit Assess 156:361–375
Chen WY, John JAC, Lin CH, Chang CY (2007) Expression pattern of metallothionein, MTF-1 nuclear translocation, and its dna‐binding activity in zebrafish (Danio rerio) induced by zinc and cadmium. Environ Toxicol Chem 26(1):110–117. https://doi.org/10.1016/0306-4492(79)90071-6
Knapen D, Reynders H, Bervoets L, Verheyen E, Blust R (2007) Metallothionein gene and protein expression as a biomarker for metal pollution in natural gudgeon populations. Aquat Toxicol 82(3):163–172
Darwish WS, Atia AS, Khedr MH, Eldin WFS (2018) Metal contamination in quail meat: residues, sources, molecular biomarkers, and human health risk assessment. Environ Sci Pollut Res 25:20106–20115
Secombes CJ, Wang T, Hong S et al (2001) Cytokines and innate immunity of fish. Dev Comp Immunol 25(8–9):713–723
Bolognesi C, Hayashi M (2011) Micronucleus assay in aquatic animals. Mutagenesis 26(1):205–213
Okonkwo JC, Obiakor MO, Nnabude PC (2011) Micronuclei profile: an index of chromosomal aberrations in fresh water fishes (Synodontis clarias and Tilapia nilotica). Online J Anim Feed Res 1(1):40–45
Roberts RJ, Agius C, Saliba C, Bossier P, Sung YY (2010) Heat shock proteins (chaperones) in fish and Shellfish and their potential role in relation to fish health: a review. J Fish Dis 33(10):789–801
Carfi M, Gennari A, Malerba I et al (2007) In vitro tests to evaluate immunotoxicity: a preliminary study. Toxicology 229(1–2):11–22
Ma J, Li Y, Wu M, Zhang C, Che Y, Li W, Li X (2018) Serum immune responses in common carp (Cyprinus carpio L.) to paraquat exposure: the traditional parameters and circulating microRNAs. Fish Shellfish Immunol 76:133–142
Magnadóttir B (2006) Innate immunity of fish (overview). Fish Shellfish Immunol 20(2):137–151
Sorg O (2004) Oxidative stress: a theoretical model or a biological reality? C R Biol 327(7):649–662
Tort L, Balasch JC, Mackenzie S (2003) Fish immune system. A crossroads between innate and adaptive responses. Inmunología 22(3):277–286
Sherif AH, Abdellatif JI, Elsiefy MM, Gouda MY, Mahmoud AE (2022) Occurrence of infectious Streptococcus agalactiae in the farmed Nile tilapia. Egypt J Aquat Biol Fish 26(3):403–432. https://doi.org/10.21608/ejabf.2022.243162
Sherif AH, Prince A, Adel Seida A, Saad Sharaf M, Eldessouki EA, Harfoush MA (2022) Moringa oleifera mitigates oxytetracycline stress in Oreochromis niloticus. Aquac Res 53(5):1790–1799. https://doi.org/10.1111/are.15707
Sherif AH, Kassab AS (2023) Multidrug-resistant Aeromonas bacteria prevalence in Nile tilapia broodstock. BMC Microbiol 23(1):80. https://doi.org/10.1186/s12866-023-02827-8
Abdel-Tawwab M, El-Sayed GO, Shady SH (2016) Growth, biochemical variables, and zinc bioaccumulation in Nile tilapia, Oreochromis niloticus (L.) as affected by water-born zinc toxicity and exposure period. Int Aquat Res 8(3):197–206
Harikrishnan R, Kim JS, Kim MC, Balasundaram C, Heo MS (2011) Lactuca indica extract as feed additive enhances immunological parameters and Disease resistance in Epinephelus bruneus to Streptococcus iniae. Aquaculture 318(1–2):43–47
Skrzep-Poloczek B, Poloczek J, Chełmecka E et al (2020) The oxidative stress markers in the erythrocytes and heart muscle of obese rats: relate to a high-fat diet but not to DJOS bariatric Surgery. Antioxidants 9(2):183. https://doi.org/10.3390/antiox9020183
Baby J, Raj JS, Biby ET, Sankarganesh P, Jeevitha MV, Ajisha SU, Rajan S. S (2010) Toxic effect of heavy metals on aquatic environment. Int J Biol Chem Sci 4(4):939–952
He W, Ai K, Ren X, Wang S, Lu L (2017) Inorganic layered ion-exchangers for decontamination of toxic metal ions in aquatic systems. J Mater Chem A 5(37):19593–19606
Funding
Funding Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
All authors equally contributed to this work. All authors analysed and interpreted the data regarding gene expression and enzymes. All authors performed the experimental study and were major contributors to writing the manuscript. All authors read, reviewed and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval
The above described methodology was approved by the Ethics Committee at the Animal Health Research Institute and European Union directive 2010/63UE, and all methods were carried out in accordance with relevant guidelines and regulations. This study is reported in accordance with ARRIVE guidelines (https://arriveguidelines.org). This paper does not contain any studies with human participants by any of the authors. No specific permissions were required for access to the artificial pond in wet laboratory Animal Health Research Institute, Kafrelsheikh, Egypt. The field studies did not involve endangered or protected species.
Consent to participate
Not applicable.
Consent to publish
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sherif, A.H., Okasha, L.A., Kassab, A.S. et al. Long-term exposure to lead nitrate and zinc sulfate Nile tilapia impact the Aeromonas hydrophila treatment. Mol Biol Rep 51, 71 (2024). https://doi.org/10.1007/s11033-023-09033-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11033-023-09033-9