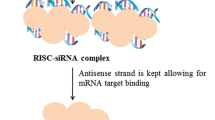
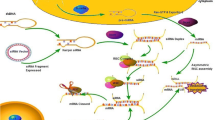
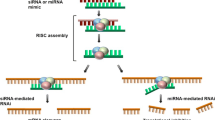
Abstract
Advancements in the clinical applications of small interfering RNA (siRNA) in cancer therapy have opened up new possibilities for precision medicine. siRNAs, as powerful genetic tools, have shown potential in targeting and suppressing the expression of specific genes associated with cancer progression. Their effectiveness has been further enhanced by incorporating them into nanoparticles, which protect siRNAs from degradation and enable targeted delivery. However, despite these promising developments, several challenges persist in the clinical translation of siRNA-based cancer therapy. This comprehensive review explores the progress and challenges associated with the clinical applications of siRNA in cancer therapy. This review highlights the use of siRNA-loaded nanoparticles as an effective delivery system for optimizing siRNA efficacy in various types of carcinomas and the potential of siRNA-based therapy as a genetic approach to overcome limitations associated with conventional chemotherapeutic agents, including severe drug toxicities and organ damage. Moreover, it emphasizes on the key challenges, including off-target effects, enzymatic degradation of siRNAs in serum, low tumor localization, stability issues, and rapid clearance from circulation that need to be addressed for successful clinical development of siRNA-based cancer therapy. Despite these challenges, the review identifies significant avenues for advancing siRNA technology from the laboratory to clinical settings. The ongoing progress in siRNA-loaded nanoparticles for cancer treatment demonstrates potential antitumor activities and safety profiles. By understanding the current state of siRNA-based therapy and addressing the existing challenges, we aim to pave the way for translating siRNA technology into effective oncologic clinics as an improved treatment options for cancer patients.


Similar content being viewed by others
Data availability
No Data associated in the manuscript.
References
Mattiuzzi C, Lippi G (2019) Current cancer epidemiology. J Epidemiol global health 9(4):217
Rabiee F, Eghbalifard N, Fathi M, Rajabi H, Riazi SS (2023) Evaluation of the potential of the MicroRNAs to predict chemotherapy resistance in breast cancer patients: a systemic review with meta-analysis. Int J Sci Res Dent Med Sci 5(3):135–140.
Kumar M, Eshwaraiah CB, KP A, Rudrappa SM (2023) Correlation of tumor-infiltrating lymphocytes with tumor staging and grading in breast carcinomas: a retrospective study. Int J Sci Res Dent Med Sci 5(1):16–20.
Guo W, Chen W, Yu W, Huang W, Deng W (2013) Small interfering RNA-based molecular therapy of cancers. Chin J cancer 32(9):488
Khan P, Siddiqui JA, Lakshmanan I, Ganti AK, Salgia R, Jain M, Batra SK, Nasser MW (2021) RNA-based therapies: a cog in the wheel of lung cancer defense. Mol Cancer 20(1):1–24
Lee SJ, Kim MJ, Kwon IC, Roberts TM (2016) Delivery strategies and potential targets for siRNA in major cancer types. Adv Drug Deliv Rev 104:2–15
Roscigno G, Scognamiglio I, Ingenito F, Chianese RV, Palma F, Chan A, Condorelli G (2020) Modulating the crosstalk between the tumor and the microenvironment using SiRNA: a flexible strategy for breast cancer treatment. Cancers 12(12):3744
Hattab D, Gazzali AM, Bakhtiar A (2021) Clinical advances of siRNA-based nanotherapeutics for cancer treatment. Pharmaceutics 13(7):1009
Salehi Kahrizsangi F, Mehrafar N, Pezhman Ghadami F, Rabiee F, Shariati Y (2022) Evaluation of the clinical outcome of nab-paclitaxel on multiple primary malignancies: a systematic review and meta-analysis. Int J Sci Res Dent Med Sci 4(4):183–190.
Cuciniello R, Filosa S, Crispi S (2021) Novel approaches in cancer treatment: preclinical and clinical development of small non-coding RNA therapeutics. J Experimental Clin Cancer Res 40(1):1–9
Narasipura EA, VanKeulen-Miller R, Ma Y, Fenton OS (2023) Ongoing clinical trials of nonviral siRNA therapeutics. Bioconjug Chem 34(7):1177–1197
Rahmani S, Rikhtechi P, Rasaneh S, Sheikholislam Z, Shahhosseini S (2017) Development of DOTA-Rituximab to be labeled with 90Y for Radioimmunotherapy of B-cell Non-Hodgkin Lymphoma. Iran J Pharm Res 16(2):619
Ozcan G, Ozpolat B, Coleman RL, Sood AK, Lopez-Berestein G (2015) Preclinical and clinical development of siRNA-based therapeutics. Adv Drug Deliv Rev 87:108–119
Mirzaei S, Gholami MH, Ang HL, Hashemi F, Zarrabi A, Zabolian A, Hushmandi K, Delfi M, Khan H, Ashrafizadeh M, Sethi G (2021) Pre-clinical and clinical applications of small interfering RNAs (siRNA) and co-delivery systems for pancreatic cancer therapy. Cells 10(12):3348
Juliano RL (2016) The delivery of therapeutic oligonucleotides. Nucleic Acids Res 44(14):6518–6548
Jain D, Prajapati SK, Jain A, Singhal R (2023) Nano-formulated siRNA-based therapeutic approaches for cancer therapy. Nano Trends 1:100006
Zhang MM, Bahal R, Rasmussen TP, Manautou JE, Zhong XB (2021) The growth of siRNA-based therapeutics: updated clinical studies. Biochem Pharmacol 189:114432
Huang Y, Hong J, Zheng S, Ding Y, Guo S, Zhang H, Zhang X, Du Q, Liang Z (2011) Elimination pathways of systemically delivered siRNA. Mol Ther 19(2):381–385
Keshavarz M, Asadi MH (2022) LncRNA ES3 is upregulated in High-Grade CRC and its expression is elevated along with increasing tumor size. J Genetic Resour 8(1):7–15
Wang J, Lu Z, Wientjes MG, Au JL (2010) Delivery of siRNA therapeutics: barriers and carriers. AAPS J 12:492–503
Friedrich M, Aigner A (2022) Therapeutic siRNA: state-of-the-art and future perspectives. BioDrugs 36(5):549–571
Hashemi A, Bigdeli R, Shahnazari M, Oruji F, Fattahi S, Panahnejad E, Ghadri A, Movahedi-Asl E, Mahdavi-Ourtakand M, Asgary V, Baghbani-Arani F (2021) Evaluation of inflammasome activation in peripheral blood mononuclear cells of hemodialysis treated patients with glomerulonephritis. Iran J Pharm Res 20(3):609
Kanasty RL, Whitehead KA, Vegas AJ, Anderson DG (2012) Action and reaction: the biological response to siRNA and its delivery vehicles. Mol Ther 20(3):513–524
Blanco E, Shen H, Ferrari M (2015) Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat Biotechnol 33(9):941–951
Babu A, Muralidharan R, Amreddy N, Mehta M, Munshi A, Ramesh R (2016) Nanoparticles for siRNA-based gene silencing in tumor therapy. IEEE Trans Nanobiosci 15(8):849–863
Kozani PS, Shabani S (2021) Adverse events and Side Effects of chimeric Antigen receptor (CAR) T cell therapy in patients with hematologic malignancies. Trends in Medical Sciences 1(1):e116301
Yu C, Li L, Hu P, Yang Y, Wei W, Deng X, Wang L, Tay FR, Ma J (2021) Recent advances in stimulus-responsive nanocarriers for gene therapy. Adv Sci 8(14):2100540
Sarisozen C, Salzano G, Torchilin VP (2015) Recent advances in siRNA delivery. Biomol concepts 6(5–6):321–341
Gao K, Huang L (2009) Nonviral methods for siRNA delivery. Mol Pharm 6(3):651–658
Slade N (2001) Viral vectors in gene therapy. Periodicum Biologorum 103(2):139–144
Mingozzi F, High KA (2013) Immune responses to AAV vectors: overcoming barriers to successful gene therapy. Blood The Journal of the American Society of Hematology 122(1):23–36
Butt MH, Zaman M, Ahmad A, Khan R, Mallhi TH, Hasan MM, Khan YH, Hafeez S, Massoud EE, Rahman MH, Cavalu S (2022) Appraisal for the potential of viral and nonviral vectors in gene therapy: a review. Genes 13(8):1370
Taha EA, Lee J, Hotta A (2022) Delivery of CRISPR-Cas tools for in vivo genome editing therapy: trends and challenges. J Controlled Release 342:345–361
Sayed N, Allawadhi P, Khurana A, Singh V, Navik U, Pasumarthi SK, Khurana I, Banothu AK, Weiskirchen R, Bharani KK (2022) Gene therapy: comprehensive overview and therapeutic applications. Life Sci 294:120375
Van Hoeck J, Braeckmans K, De Smedt SC, Raemdonck K (2022) Non-viral siRNA delivery to T cells: Challenges and opportunities in cancer immunotherapy. Biomaterials 286:121510
Leong EW, Ge R (2022) Lipid nanoparticles as Delivery Vehicles for inhaled therapeutics. Biomedicines 10(9):2179
Kalita T, Dezfouli SA, Pandey LM, Uludag H (2022) siRNA functionalized lipid nanoparticles (LNPs) in management of Diseases. Pharmaceutics 14(11):2520
Sufianov A, Beilerli A, Kudriashov V, Ilyasova T, Wenjie B, Beylerli O (2023) Advances in transdermal siRNAs delivery: a review of current research progress. Non-coding RNA Research 8(3):392–400
Mohamadi N, Kazemi SM, Mohammadian M, Milani AT, Moradi Y, Yasemi M, Tabrizi MM, Shahmabadi HE, Khiyavi AA (2017) Toxicity of cisplatin-loaded poly butyl cyanoacrylate nanoparticles in a brain cancer cell line: anionic polymerization results. Asian Pac J Cancer Prev 18(3):629
Oruji F, Baghbani Arani F, Mahdavi Ortakand M (2018) Evaluation of the gene expression of IL-1β and Casp-1 related to inflammation process in glomerulonephritis patients. J Anim Environ 10(3):477–482
Zhu H, Zheng J, Oh XY, Chan CY, Low BQ, Tor JQ, Jiang W, Ye E, Loh XJ, Li Z (2023) Nanoarchitecture-Integrated Hydrogel Systems toward Therapeutic Applications. ACS Nano 17(9):7953–7978
Niculescu AG, Bîrcă AC, Grumezescu AM (2021) New applications of lipid and polymer-based nanoparticles for nucleic acids delivery. Pharmaceutics 13(12):2053
Cai X, Dou R, Guo C, Tang J, Li X, Chen J, Zhang J (2023) Cationic polymers as transfection reagents for nucleic acid delivery. Pharmaceutics 15(5):1502
Jadid MF, Shademan B, Chavoshi R, Seyyedsani N, Aghaei E, Taheri E, Goleij P, Hajazimian S, Karamad V, Behroozi J, Sabet MN, Isazadeh A, Baradaran B (2021) Enhanced anticancer potency of hydroxytyrosol and curcumin by PLGA‐PAA nano‐encapsulation on PANC‐1 pancreatic cancer cell line. Environ Toxicol 36(6):1043–1051.
Chen Y, Sun B, Marcella C (2023) Evaluation of the diagnostic accuracy of superparamagnetic iron oxide nanoparticles on breast cancer: a systematic review and meta-analysis. Int J Sci Res Dent Med Sci 5(1):27–34.
Yadav K, Sahu KK, Gnanakani SP, Sure P, Vijayalakshmi R, Sundar VD, Sharma V, Antil R, Jha M, Minz S, Bagchi A (2023) Biomedical applications of nanomaterials in the advancement of nucleic acid therapy: mechanistic challenges, delivery strategies, and therapeutic applications. Int J Biol Macromol 241:124582
Gholami L, Ivari JR, Nasab NK, Oskuee RK, Sathyapalan T, Sahebkar A (2023) Recent advances in lung cancer therapy based on nanomaterials: a review. Curr Med Chem 30(3):335–355
Ebrahimifar M, Roudsari MH, Kazemi SM, Shahmabadi HE, Kanaani L, Alavi SA, Vasfi MI (2017) Enhancing effects of curcumin on cytotoxicity of paclitaxel, methotrexate and vincristine in gastric cancer cells. Asian Pac J Cancer Prev 18(1):65
Draz MS, Fang BA, Zhang P, Hu Z, Gu S, Weng KC, Gray JW, Chen FF (2014) Nanoparticle-mediated systemic delivery of siRNA for treatment of cancers and viral infections. Theranostics 4(9):872
Subhan MA, Torchilin VP (2020) siRNA based drug design, quality, delivery and clinical translation. Nanomed: Nanotechnol Biol Med 29:102239
Erazo-Oliveras A, Muthukrishnan N, Baker R, Wang TY, Pellois JP (2012) Improving the endosomal escape of cell-penetrating peptides and their cargos: strategies and challenges. Pharmaceuticals 5(11):1177–1209
Mohamed RJ, Aldulaimi A, Aowda SA (2022) Synthesized of new alkaloid compounds and study their anticancer activity. AIP Conf Proc 2660(1):1.
Stiltner J, McCandless K, Zahid M (2021) Cell-penetrating peptides: applications in tumor diagnosis and therapeutics. Pharmaceutics 13(6):890
Mitchell MJ, Billingsley MM, Haley RM, Wechsler ME, Peppas NA, Langer R (2021) Engineering precision nanoparticles for drug delivery. Nat Rev Drug Discovery 20(2):101–124
Zare H, Ahmadi S, Ghasemi A, Ghanbari M, Rabiee N, Bagherzadeh M, Karimi M, Webster TJ, Hamblin MR, Mostafavi E (2021) Carbon nanotubes: Smart drug/gene delivery carriers. Int J Nanomed 16:1681
Eş I, Malfatti-Gasperini AA, de la Torre LG (2022) The diffusion-driven microfluidic process to manufacture lipid-based nanotherapeutics with stealth properties for siRNA delivery. Colloids Surf B 215:112476
Akbarzadeh A, Rezaei-Sadabady R, Davaran S, Joo SW, Zarghami N, Hanifehpour Y, Samiei M, Kouhi M, Nejati-Koshki K (2013) Liposome: classification, preparation, and applications. Nanoscale Res Lett 8(1):1–9
Liu M, Wang L, Lo Y, Shiu SC, Kinghorn AB, Tanner JA (2022) Aptamer-enabled nanomaterials for therapeutics, drug targeting and imaging. Cells 11(1):159
Nakhaei P, Margiana R, Bokov DO, Abdelbasset WK, Jadidi Kouhbanani MA, Varma RS, Marofi F, Jarahian M, Beheshtkhoo N (2021) Liposomes: structure, biomedical applications, and stability parameters with emphasis on cholesterol. Front Bioeng Biotechnol 9:748
Tong Q, Qiu N, Ji J, Ye L, Zhai G (2020) Research progress in bioinspired drug delivery systems. Expert Opin Drug Deliv 17(9):1269–1288
Ståhl AL, Johansson K, Mossberg M, Kahn R, Karpman D (2019) Exosomes and microvesicles in normal physiology, pathophysiology, and renal diseases. Pediatr Nephrol 34:11–30
Kurakula H, Vaishnavi S, Sharif MY, Ellipilli S (2023) Emergence of small interfering RNA-Based gene drugs for various Diseases. ACS omega 8(23):20234–20250
Jamali S, Nouhravesh M (2023) Evaluation of the clinical outcome of carbon nanoparticles on thyroid cancer: a systematic review and meta-analysis. Int J Sci Res Dent Med Sci 5(1):48-56.
Zarrintaj P, Ganjali MR, Salmankhani A, Mashhadzadeh AH, Munir MT, Salehnia F, Rezapour M, Habibzadeh S, Saeb MR (2023) Carboxymethylated polysaccharides in drug delivery. Tailor-Made Polysaccharides Drug Deliv 63–81.
Jasinski D, Haque F, Binzel DW, Guo P (2017) Advancement of the emerging field of RNA nanotechnology. ACS Nano 11(2):1142–1164
Geary C, Chworos A, Verzemnieks E, Voss NR, Jaeger L (2017) Composing RNA nanostructures from a syntax of RNA structural modules. Nano Lett 17(11):7095–7101
Pi F, Binzel DW, Lee TJ, Li Z, Sun M, Rychahou P, Li H, Haque F, Wang S, Croce CM, Guo B (2018) Nanoparticle orientation to control RNA loading and ligand display on extracellular vesicles for cancer regression. Nat Nanotechnol 13(1):82–89
Shu D, Li H, Shu Y, Xiong G, Carson WE III, Haque F, Xu R, Guo P (2015) Systemic delivery of anti-miRNA for suppression of triple negative breast cancer utilizing RNA nanotechnology. ACS Nano 9(10):9731–9740
Fathi M, Riazi SS, Pourdamghan N (2023) Triple-negative breast cancer therapy using RNA nanoparticles targeting stem cell markers with anti-miRNA: a systematic review and meta-analysis. Int J Sci Res Dent Med Sci 5(2):96–101.
Bajracharya R, Song JG, Patil BR, Lee SH, Noh HM, Kim DH, Kim GL, Seo SH, Park JW, Jeong SH, Lee CH (2022) Functional ligands for improving anticancer drug therapy: current status and applications to drug delivery systems. Drug Delivery 29(1):1959–1970
Mosayebnia M, Rezaeianpour S, Rikhtechi P, Hajimahdi Z, Beiki D, Kobarfard F, Amini M, Abdi K, Shahhosseini S (2018) Novel and efficient method for solid phase synthesis of urea-containing peptides targeting prostate specific membrane antigen (PSMA) in comparison with current methods. Iran J Pharm Res 17(3):917
Ebrahimifar M, Nili-Ahmadabadi A, Akbarzadeh A, Shahemabadi HE, Hasanzadegan M, Moradi-Sardareh H, Madadizadeh H, Rezaee-Diyan J (2017) Preparation, characterization and cytotoxic effects of pegylated nanoliposomal containing carboplatin on ovarian cancer cell lines. Indian J Clin Biochem 32:230–234
Kaboli PJ, Shabani S, Sharma S, Nasr MP, Yamaguchi H, Hung MC (2022) Shedding light on triple-negative breast cancer with Trop2-targeted antibody-drug conjugates. Am J cancer Res 12(4):1671
Mehralizadeh H, Nazari A, Oruji F, Roostaie M, Hosseininozari G, Yazdani O, Esbati R, Roudini K (2023) Cytokine sustained delivery for cancer therapy; special focus on stem cell-and biomaterial-based delivery methods. Pathology-Research and Practice 247:154528
Subhan MA, Torchilin VP (2019) Efficient nanocarriers of siRNA therapeutics for cancer treatment. Translational Res 214:62–91
Neeleman RA, Wensink D, Wagenmakers MA, Mijnhout GS, Friesema EC, Langendonk JG (2020) Diagnostic and therapeutic strategies for porphyrias. Neth J Med 78(4):149–160
Frishberg Y, Deschenes G, Cochat P, Magen D, Groothoff J, Hulton SA, Harambat J, vant Hoff W, Hoppe B, Lieske* JC, McGregor TL (2019) Mp12-14 safety and efficacy study of Lumasiran, an investigational RNA interference (RNAI) therapeutic, in Adult and Pediatric patients with primary hyperoxaluria type 1 (PH1). J Urol 201(Supplement 4):e174
Sheridan C (2019) PCSK9-gene-silencing, cholesterol-lowering drug impresses. Nat Biotechnol 37(12):1385–1388
Urits I, Swanson D, Swett MC, Patel A, Berardino K, Amgalan A, Berger AA, Kassem H, Kaye AD, Viswanath O (2020) A review of patisiran (ONPATTRO®) for the treatment of polyneuropathy in people with hereditary transthyretin amyloidosis. Neurol Ther 9:301–315
Paul A, Muralidharan A, Biswas A, Kamath BV, Joseph A, Alex AT (2022) siRNA therapeutics and its challenges: recent advances in effective delivery for cancer therapy. OpenNano 7:100063
Smith ES, Whitty E, Yoo B, Moore A, Sempere LF, Medarova Z (2022) Clinical applications of short non-coding RNA-based therapies in the era of precision medicine. Cancers 14(6):1588
Naito Y, Yoshimura J, Morishita S, Ui-Tei K (2009) siDirect 2.0: updated software for designing functional siRNA with reduced seed-dependent off-target effect. BMC Bioinformatics 10(1):1–8
Sohrab SS, El-Kafrawy SA, Mirza Z, Kamal MA, Azhar EI (2018) Design and delivery of therapeutic siRNAs: application to MERS-coronavirus. Curr Pharm Design 24(1):62–77
Hajebi S, Yousefiasl S, Rahimmanesh I, Dahim A, Ahmadi S, Kadumudi FB, Rahgozar N, Amani S, Kumar A, Kamrani E, Rabiee M (2022) Genetically Engineered viral vectors and Organic-Based non‐viral nanocarriers for drug delivery applications. Adv Healthc Mater 11(20):2201583
Kamola PJ, Nakano Y, Takahashi T, Wilson PA, Ui-Tei K (2015) The siRNA non-seed region and its target sequences are auxiliary determinants of off-target effects. PLoS Comput Biol 11(12):e1004656
Ryther RC, Flynt AS, Phillips J, Patton JG (2005) siRNA therapeutics: big potential from small RNAs. Gene Ther 12(1):5–11
Kim DH, Rossi JJ (2007) Strategies for silencing human disease using RNA interference. Nat Rev Genet 8(3):173–184
Fakhr E, Zare F, Teimoori-Toolabi L (2016) Precise and efficient siRNA design: a key point in competent gene silencing. Cancer Gene Ther 23(4):73–82
Janas MM, Schlegel MK, Harbison CE, Yilmaz VO, Jiang Y, Parmar R, Zlatev I, Castoreno A, Xu H, Shulga-Morskaya S, Rajeev KG (2018) Selection of GalNAc-conjugated siRNAs with limited off-target-driven rat hepatotoxicity. Nat Commun 9(1):723
Song X, Wang X, Ma Y, Liang Z, Yang Z, Cao H (2017) Site-specific modification using the 2′-methoxyethyl group improves the specificity and activity of siRNAs. Mol Therapy-Nucleic Acids 9:242–250
Godinho B, Khvorova A (2019) The era of RNA interference medicines: the clinical landscape of synthetic gene silencing drugs. Saúde & Tecnologia 21:5–17
Kim JH, Kim YS, Park K, Kang E, Lee S, Nam HY, Kim K, Park JH, Chi DY, Park RW, Kim IS (2008) Self-assembled glycol chitosan nanoparticles for the sustained and prolonged delivery of antiangiogenic small peptide drugs in cancer therapy. Biomaterials 29(12):1920–1930
Han HD, Mangala LS, Lee JW, Shahzad MM, Kim HS, Shen D, Nam EJ, Mora EM, Stone RL, Lu C, Lee SJ (2010) Targeted gene silencing using RGD-labeled chitosan nanoparticles. Clin Cancer Res 16(15):3910–3922
Lam JK, Chow MY, Zhang Y, Leung SW (2015) siRNA versus miRNA as therapeutics for gene silencing. Mol Therapy-Nucleic Acids 4:e252
Suñé-Pou M, Limeres MJ, Moreno-Castro C, Hernández-Munain C, Suñé-Negre JM, Cuestas ML, Suñé C (2020) Innovative therapeutic and delivery approaches using nanotechnology to correct splicing defects underlying disease. Front Genet 11:731
Larkins NG, Liu ID, Willis NS, Craig JC, Hodson EM (2020) Non-corticosteroid immunosuppressive medications for steroid‐sensitive nephrotic syndrome in children. Cochrane Database of Systematic Reviews 2020(4):CD002290
Huang J, Xiao K (2022) Nanoparticles-based strategies to improve the delivery of therapeutic small interfering RNA in precision oncology. Pharmaceutics 14(8):1586
Guo P, Coban O, Snead NM, Trebley J, Hoeprich S, Guo S, Shu Y (2010) Engineering RNA for targeted siRNA delivery and medical application. Adv Drug Deliv Rev 62(6):650–666
Yu Z, Ye J, Pei X, Sun L, Liu E, Wang J, Huang Y, Lee SJ, He H (2018) Improved method for synthesis of low molecular weight protamine–siRNA conjugate. Acta Pharm Sinica B 8(1):116–126
Fathi N, Saadati A, Hasanzadeh M, Samiei M (2021) Chemical binding of pyrrolidinyl peptide nucleic acid (acpcPNA-T9) probe with AuNPs toward label‐free monitoring of miRNA‐21: a novel biosensing platform for biomedical analysis and POC diagnostics. J Mol Recognit 34(8):e2893
Fath MK, Naderi M, Hamzavi H, Ganji M, Shabani S, Khalesi B, Pourzardosht N, Hashemi ZS, Khalili S (2022) Molecular Mechanisms and therapeutic effects of different vitamins and minerals in COVID-19 patients. J Trace Elem Med Biol 73:127044
Selvam C, Mutisya D, Prakash S, Ranganna K, Thilagavathi R (2017) Therapeutic potential of chemically modified si RNA: recent trends. Chem Biol Drug Des 90(5):665–678
Fu Z, Li S, Han S, Shi C, Zhang Y (2022) Antibody drug conjugate: the “biological missile” for targeted cancer therapy. Signal Transduct Target Therapy 7(1):93
Servidei T, Lucchetti D, Navarra P, Sgambato A, Riccardi R, Ruggiero A (2021) Cell-of-origin and genetic, epigenetic, and microenvironmental factors contribute to the intra-tumoral heterogeneity of pediatric intracranial ependymoma. Cancers 13(23):6100
Easwaran H, Tsai HC, Baylin SB (2014) Cancer epigenetics: tumor heterogeneity, plasticity of stem-like states, and drug resistance. Mol Cell 54(5):716–727
Kumar K, Rani V, Mishra M, Chawla R (2022) New paradigm in combination therapy of siRNA with chemotherapeutic drugs for effective cancer therapy. Curr Res Pharmacol Drug Discovery 3:100103
Shabani S, Moghadam MF, Gargari SL (2021) Isolation and characterization of a novel GRP78-specific single-chain variable fragment (scFv) using ribosome display method. Med Oncol 38(9):115
Ponomarev A, Gilazieva Z, Solovyeva V, Allegrucci C, Rizvanov A (2022) Intrinsic and extrinsic factors impacting cancer stemness and tumor progression. Cancers 14(4):970
Yu Z, Pestell TG, Lisanti MP, Pestell RG (2012) Cancer stem cells. Int J Biochem Cell Biol 44(12):2144–2151
Abballe L, Spinello Z, Antonacci C, Coppola L, Miele E, Catanzaro G, Miele E (2023) Nanoparticles for drug and gene delivery in pediatric brain tumors’ cancer stem cells: current knowledge and future perspectives. Pharmaceutics 15(2):505
Zong X, Nephew KP (2019) Ovarian cancer stem cells: role in metastasis and opportunity for therapeutic targeting. Cancers 11(7):934
Imai K, Taniguchi H (2022) Therapeutic siRNA targeting the cancer cell stemness regulator PRDI-BF1 and RIZ domain zinc finger protein 14. Proc Japan Acad Ser B 98(7):325–335
Chen X, Mangala LS, Rodriguez-Aguayo C, Kong X, Lopez-Berestein G, Sood AK (2018) RNA interference-based therapy and its delivery systems. Cancer Metastasis Rev 37:107–124
Dong Y, Siegwart DJ, Anderson DG (2019) Strategies, design, and chemistry in siRNA delivery systems. Adv Drug Deliv Rev 144:133–147
Albertsen CH, Kulkarni JA, Witzigmann D, Lind M, Petersson K, Simonsen JB (2022) The role of lipid components in lipid nanoparticles for vaccines and gene therapy. Adv Drug Deliv Rev 188:114416
Dana H, Chalbatani GM, Mahmoodzadeh H, Karimloo R, Rezaiean O, Moradzadeh A, Mehmandoost N, Moazzen F, Mazraeh A, Marmari V, Ebrahimi M (2017) Molecular mechanisms and biological functions of siRNA. Int J biomedical Sci 13(2):48
Zhao Y, Wang W, Guo S, Wang Y, Miao L, Xiong Y, Huang L (2016) PolyMetformin combines carrier and anticancer activities for in vivo siRNA delivery. Nat Commun 7(1):11822
Tatiparti K, Sau S, Kashaw SK, Iyer AK (2017) siRNA delivery strategies: a comprehensive review of recent developments. Nanomaterials 7(4):77
Zheng N, Luo X, Zhang Z, Wang A, Song W (2021) Cationic polyporphyrins as siRNA delivery vectors for photodynamic and gene synergistic anticancer therapy. ACS Appl Mater Interfaces 13(23):27513–27521
Sajid MI, Moazzam M, Kato S, Yeseom Cho K, Tiwari RK (2020) Overcoming barriers for siRNA therapeutics: from bench to bedside. Pharmaceuticals 13(10):294
Plath K, Srivastava D, Alvarez-Buylla A, Tanaka EM, Kriegstein AR (2012) Stem cells in the land of the rising Sun: ISSCR 2012. Cell Stem Cell 11(5):607–614
Zare M, Pemmada R, Madhavan M, Shailaja A, Ramakrishna S, Kandiyil SP, Donahue JM, Thomas V (2022) Encapsulation of miRNA and siRNA into nanomaterials for Cancer therapeutics. Pharmaceutics 14(8):1620
Liu Z, Parveen N, Rehman U, Aziz A, Sheikh A, Abourehab MA, Guo W, Huang J, Wang Z, Kesharwani P (2023) Unravelling the enigma of siRNA and aptamer mediated therapies against pancreatic cancer. Mol Cancer 22(1):1–22
Rinoldi C, Zargarian SS, Nakielski P, Li X, Liguori A, Petronella F, Presutti D, Wang Q, Costantini M, De Sio L, Gualandi C (2021) Nanotechnology-assisted RNA delivery: from nucleic acid therapeutics to COVID‐19 vaccines. Small Methods 5(9):2100402
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure of potential conflicts of interest
The authors have no conflict of interest with anybody or any competing interests.
Research involving human participants and/or animals
None applicable.
Informed consent
None applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Isazadeh, H., Oruji, F., Shabani, S. et al. Advances in siRNA delivery approaches in cancer therapy: challenges and opportunities. Mol Biol Rep 50, 9529–9543 (2023). https://doi.org/10.1007/s11033-023-08749-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-023-08749-y