Abstract
Lifestyle modification can lead to numerous health issues closely associated with sleep. Sleep deprivation and disturbances significantly affect inflammation, immunity, neurodegeneration, cognitive depletion, memory impairment, neuroplasticity, and insulin resistance. Sleep significantly impacts brain and memory formation, toxin excretion, hormonal function, metabolism, and motor and cognitive functions. Sleep restriction associated with insulin resistance affects these functions by interfering with the insulin signalling pathway, neurotransmission, inflammatory pathways, and plasticity of neurons. So, in this review, We discuss the evidence that suggests that neurodegeneration occurs via sleep and is associated with insulin resistance, along with the insulin signalling pathways involved in neurodegeneration and neuroplasticity, while exploring the role of hormones in these conditions.
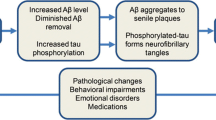
Graphical Abstract

Highlights
-
Insulin resistance increases the accumulation of amyloid β, tau, and synuclein in neurons, leading to neurodegeneration.
-
The insulin signalling pathway has a potential role in sleep deprivation-induced insulin resistance.
-
Neurohormones that play a role in the sleep/wake cycle modulate insulin sensitivity, metabolism, and neurodegeneration.





Similar content being viewed by others
Data availability
No data associated with the manuscript.
References
Heyde I, Oster H (2022) Induction of internal circadian desynchrony by misaligning zeitgebers. Sci Rep 12(1):1–10. https://doi.org/10.1038/s41598-022-05624-x
Koch CE et al (2020) Circadian regulation of hedonic appetite in mice by clocks in dopaminergic neurons of the VTA. Nat Commun 11(1):1–11. https://doi.org/10.1038/s41467-020-16882-6
Krueger JM, Nguyen JT, Dykstra-Aiello CJ, Taishi P (2019) Local sleep. Sleep Med Rev 43:14–21. https://doi.org/10.1016/j.smrv.2018.10.001
Barbato G (2021) REM sleep: an unknown indicator of sleep quality. Int J Environ Res Public Health 18:24. https://doi.org/10.3390/ijerph182412976
Borges CR, Poyares D, Piovezan R, Nitrini R, Brucki S (2019) “Alzheimer ’ s disease and sleep disturbances : a review,”. pp 815–824
Gupta R et al (2020) Changes in sleep pattern and sleep quality during COVID-19 lockdown. Indian J Psychiatry 62(4):370–378. https://doi.org/10.4103/psychiatry.IndianJPsychiatry
Pinto J et al (2020) Sleep quality in times of Covid-19 pandemic. Sleep Med 74:81–85. https://doi.org/10.1016/j.sleep.2020.07.012
Lissak G (2018) Adverse physiological and psychological effects of screen time on children and adolescents: literature review and case study. Environ Res 2018(164):149–157. https://doi.org/10.1016/j.envres.2018.01.015
Novozhilova M et al (2021) Features of age-related response to sleep deprivation: in vivo experimental studies. Aging 13:19108–19126. https://doi.org/10.18632/aging.203372
Pimolsri C et al (2021) Objective sleep duration and timing predicts completion of in vitro fertilization cycle. J Assist Reprod Genet 38(10):2687–2696. https://doi.org/10.1007/s10815-021-02260-8
Bishir M et al (2020) Sleep deprivation and neurological disorders. Biomed Res Int. https://doi.org/10.1155/2020/5764017
Ma Y, Liang L, Zheng F, Shi L, Zhong B, Xie W (2020) Association between sleep duration and cognitive decline. JAMA Netw Open 3(9):e2013573. https://doi.org/10.1001/jamanetworkopen.2020.13573
Overberg J, Kalveram L, Keller T, Krude H, Kühnen P, Wiegand S (2022) Interactions between nocturnal melatonin secretion, metabolism, and sleeping behavior in adolescents with obesity. Int J Obes 46(5):1051–1058. https://doi.org/10.1038/s41366-022-01077-4
Lamon S et al (2021) The effect of acute sleep deprivation on skeletal muscle protein synthesis and the hormonal environment. Physiol Rep. https://doi.org/10.14814/phy2.14660
Ahmed OG, Mahmoud GS, Samy SS, Sayed SA (2023) The protective effect of melatonin on chronic paradoxical sleep deprivation induced metabolic and memory deficit in rats. Int J Pathophysiol Pharmacol 15(3):56–74
Zhao H et al (2016) Frontal cortical mitochondrial dysfunction and mitochondria-related β-amyloid accumulation by chronic sleep restriction in mice. NeuroReport 27(12):916–922. https://doi.org/10.1097/WNR.0000000000000631
Ferraz-Bannitz R, Beraldo RA, Coelho PO, Moreira AC, Castro M, Foss-Freitas MC (2021) Circadian misalignment induced by chronic night shift work promotes endoplasmic reticulum stress activation impacting directly on human metabolism. Biology (Basel) 10(3):1–13. https://doi.org/10.3390/biology10030197
Holth JK et al (2020) The sleep-wake cycle regulates brain interstitial fluid tau in mice and CSF tau in humans. Science 363(6429):880–884. https://doi.org/10.1126/science.aav2546.The
Gottlieb E et al (2020) Regional neurodegenaration correlates with sleep-wake dysfunction after stroke. Sleep Res Soc 43(9):1–13
Feld GB et al (2016) Central nervous insulin signaling in sleep-associated memory formation and neuroendocrine regulation. Neuropsychopharmacology 41(6):1540–1550. https://doi.org/10.1038/npp.2015.312
Yamaguchi ST, Tomita J, Kume K (2022) Insulin signaling in clock neurons regulates sleep in Drosophila. Biochem Biophys Res Commun 591:44–49. https://doi.org/10.1016/j.bbrc.2021.12.100
Brzecka A et al (2020) Sleep disturbances and cognitive impairment in the course of type 2 diabetes-a possible link. Curr Neuropharmacol 19(1):78–91
Sweeney EL, Jeromson S, Hamilton DL, Brooks NE, Walshe IH (2017) Skeletal muscle insulin signaling and whole-body glucose metabolism following acute sleep restriction in healthy males. Physiol Rep 5(23):1–8. https://doi.org/10.14814/phy2.13498
Falcicchia C, Tozzi F, Arancio O, Watterson DM, Origlia N (2020) Involvement of p38 MAPK in synaptic function and dysfunction. Int J Mol Sci. https://doi.org/10.3390/ijms21165624
Ramasubbu K, Rajeswari VD, Autophagy-related ATG (2022) Impairment of insulin signaling pathway PI3K / Akt / mTOR and insulin resistance induced AGEs on diabetes mellitus and neurodegenerative diseases : a perspective review Receptor for AGEs. Mol Cell Biochem. https://doi.org/10.1007/s11010-022-04587-x
Sondrup N et al (2022) Effects of sleep manipulation on markers of insulin sensitivity : a systematic review and meta-analysis of randomized controlled trials. Sleep Med. Rev. 62:101594. https://doi.org/10.1016/j.smrv.2022.101594
Reutrakul S, Sumritsopak R, Saetung S, Chanprasertyothin S, Anothaosintawee T (2017) The relationship between sleep and glucagon-like peptide 1 in patients with abnormal glucose tolerance. J Sleep Res. https://doi.org/10.1111/jsr.12552
Park J et al (2022) Daily injection of melatonin inhibits insulin resistance induced by chronic mealtime shift. Physiol Rep 10(6):1–9. https://doi.org/10.14814/phy2.15227
Pignatelli J, De Sevilla MEF, Sperber J, Horrillo D, Medina-gomez G, Aleman IT (2022) Insulin-like growth factor i couples metabolism with circadian activity through hypothalamic orexin neurons. Intern J Mol Sci. https://doi.org/10.3390/ijms23094679
Bruni O, Ferini-Strambi L, Giacomoni E, Pellegrino P (2021) Herbal remedies and their possible effect on the GABAergic system and sleep. Nutrients 13:1–13. https://doi.org/10.3390/nu130205301
Yamada RG, Ueda HR (2020) Molecular mechanisms of REM sleep. Front Neurosci 13:1–15. https://doi.org/10.3389/fnins.2019.01402
Sakai K, Crochet S (2003) A neural mechanism of sleep and wakefulness. Sleep Biol Rhythms 1:29–42
Wang X, Bik A, De Groot ER, Luisa M, Benders MJNL, Dudink J (2023) Clinical neurophysiology feasibility of automated early postnatal sleep staging in extremely and very preterm neonates using dual-channel EEG. Clin Neurophysiol 146:55–64. https://doi.org/10.1016/j.clinph.2022.11.018
Chai Y et al (2020) Two nights of recovery sleep restores hippocampal connectivity but not episodic memory after total sleep deprivation. Sci Rep 10(1):1–11. https://doi.org/10.1038/s41598-020-65086-x
Chu C, Holst SC, Elmenhorst E, Foerges AL (2023) Total sleep deprivation increases brain age prediction reversibly in multi-site samples of young healthy adults. J Neurosci. https://doi.org/10.1523/JNEUROSCI.0790-22.2023
MacDonald KJ, Cote KA (2021) Contributions of post-learning REM and NREM sleep to memory retrieval. Sleep Med. Rev. 59:101453. https://doi.org/10.1016/j.smrv.2021.101453
Eban-rothschild A, Appelbaum L, De Lecea L (2018) Neuronal mechanisms for sleep / wake regulation and modulatory drive. Nat Publ Gr 43(5):937–952. https://doi.org/10.1038/npp.2017.294
Craig-Heller H, Ruby FN, Rolls A, Makam M, Colas D (2014) Adaptive and pathological inhibition of neuroplasticity associated with circadian rhythms and sleep. Behav Neurosci. 128(3):273–282. https://doi.org/10.1037/a0036689.Adaptive
Helakari H et al (2022) Human NREM sleep promotes brain-wide vasomotor and respiratory pulsations. J Neurosci 42(12):2503–2515. https://doi.org/10.1523/JNEUROSCI.0934-21.2022
Riemann D, Krone LB, Wulff K, Nissen C (2020) Sleep, insomnia, and depression. Neuropsychopharmacology 45(1):74–89. https://doi.org/10.1038/s41386-019-0411-y
Li W, Yang G, Gan W (2017) REM sleep selectively prunes and maintains new synapses in development and learning. Nat Neurosci 20(3):427–437. https://doi.org/10.1038/nn.4479.REM
Bruni O, Ferini-Strambi L, Giacomoni E, Pellegrino P (2021) Herbal remedies and their possible effect on the gabaergic system and sleep. Nutrients 13(2):1–13. https://doi.org/10.3390/nu13020530
Hasegawa E, Miyasaka A, Sakurai K, Cherasse Y, Li Y, Sakurai T (2022) Rapid eye movement sleep is initiated by basolateral amygdala dopamine signaling in mice. Science. 375(6584):994–1000. https://doi.org/10.1126/science.abl6618
Nelson KL, Davis JE, Corbett CF (2022) Sleep quality: an evolutionary concept analysis. Nurs Forum 57(1):144–151. https://doi.org/10.1111/nuf.12659
Erion R, DiAngelo JR, Crocker A, Sehgal A (2012) Interaction between sleep and metabolism in Drosophila with altered octopamine signaling. J Biol Chem 287(39):32406–32414. https://doi.org/10.1074/jbc.M112.360875
Benítez-king CG et al (2018) Circadian modulation of neuroplasticity by melatonin : a target in the treatment of depression. Br J Pharmacol 175:3200–3208. https://doi.org/10.1111/bph.14197
Ukraintseva YV, Kovalzon VM (2016) Circadian regulation and its disorders in Parkinson’s disease patients. part 2: experimental models, alpha-synuclein, and melatonin. Hum Physiol 42(5):559–570. https://doi.org/10.1134/S0362119716050170
Shen L, Wiley JF, Bei B (2021) Perceived daily sleep need and sleep debt in adolescents: associations with daily affect over school and vacation periods. Sleep 44(12):1–9. https://doi.org/10.1093/sleep/zsab190
Salehinejad MA, Ghanavati E, Reinders J, Kuo F, Nitsche MA (2022) Sleep-dependent upscaled excitability, saturated neuroplasticity, and modulated cognition in the human brain. Neuroscience 1:1–33
Palagini L, Geoffroy PA, Riemann D (2022) Sleep markers in psychiatry: do insomnia and disturbed sleep play as markers of disrupted neuroplasticity in mood disorders? A pro- posed model. Curr Med Chem 22:5595–5605. https://doi.org/10.2174/0929867328666211214164907
Broussard J, Brady MJ (2010) The impact of sleep disturbances on adipocyte function and lipid metabolism. Best Pract Res Clin Endocrinol Metab 24(5):763–773. https://doi.org/10.1016/j.beem.2010.08.007
Meyhöfer S et al (2022) Sleep deprivation prevents counterregulatory adaptation to recurrent hypoglycaemia. Diabetologia 65:1212–1221. https://doi.org/10.1007/s00125-022-05702-9
Stoelzel CR, Cincotta AH (2020) Circadian-timed dopamine agonist treatment reverses high-fat diet-induced diabetogenic shift in ventromedial hypothalamic glucose sensing. Endocrinol Metab. https://doi.org/10.1002/edm2.139
Oomura Y, Ono T, Ooyama H, Wayner MJ (1969) Glucose and osmosensitive neurones of the rat hypothalamus. Nature 222(5190):282–284. https://doi.org/10.1038/222282a0
Routh VH, Hao L, Santiago AM, Sheng Z, Zhou C, Chan O (2014) Hypothalamic glucose sensing : making ends meet. Front Syst Neurosci 8(December):1–13. https://doi.org/10.3389/fnsys.2014.00236
Yoon NA, Diano S (2021) Hypothalamic glucose-sensing mechanisms. Diabetologia 64:985–993. https://doi.org/10.1007/s00125-021-05395-6
So-Ngern A, Chirakalwasan N, Saetung S, Chanprasertyothin S, Thakkinstian A, Reutrakul S (2019) Effects of two-week sleep extension on glucose metabolism in chronically sleep-deprived individuals. J Clin Sleep Med 15(5):711–8
Hashempour S, Ghorbani A, Khashayar A, Olfati H (2021) Association of sleep quality with insulin resistance in obese or overweight subjects. Sleep Sci 14:75–78. https://doi.org/10.5935/1984-0063.20200084
Kontou TG, Sargent C, Roach GD (2022) A week of sleep restriction does not affect nighttime glucose concentration in healthy adult males when slow-wave sleep is maintained. Sensors. https://doi.org/10.3390/s22186962
Ness XKM, Strayer SM, Nahmod NG, Chang A, Buxton OM, Shearer GC (2019) Two nights of recovery sleep restores the dynamic lipemic response, but not the reduction of insulin sensitivity, induced by five nights of sleep restriction. Am J Physiol Regul Integr Comp Physiol 316:697–703. https://doi.org/10.1152/ajpregu.00336.2018
Zheng P et al (2020) Evaluating the effects of different sleep supplement modes in attenuating metabolic consequences of night shift work using rat model. Nat Sci Sleep 12:1053–1065
Andica C et al (2023) Neuroimaging findings related to glymphatic system alterations in older adults with metabolic syndrome. Neurobiol Dis 177:105990. https://doi.org/10.1016/j.nbd.2023.105990
Kim YK, Il-Nam K, Song J (2018) The glymphatic system in diabetes-induced dementia. Front Neurol 9:1–10. https://doi.org/10.3389/fneur.2018.00867
Reddy OC, van der Werf YD (2020) The sleeping brain: Harnessing the power of the glymphatic system through lifestyle choices. Brain Sci 10(11):1–16. https://doi.org/10.3390/brainsci10110868
Benveniste H, Liu X, Koundal S, Sanggaard S, Lee H, Wardlaw J (2019) The glymphatic system and waste clearance with brain aging: a review. Gerontology 65(2):106–119. https://doi.org/10.1159/000490349
Galli M, Hameed A, Żbikowski A, Zabielski P (2021) Aquaporins in insulin resistance and diabetes: more than channels! Redox Biol. https://doi.org/10.1016/j.redox.2021.102027
Carroll EJ, Cole WS, Seeman ET, Breen CE (2019) Partial Sleep Deprivation Activates the DNA damage response (DDR) and the senescence-associated secretory phenotype (SASP) in Aged Adult Humans. Physiol Behav 176(3):139–148. https://doi.org/10.1016/j.bbi.2015.08.024.Partial
Lee HK et al (2017) MTORC2 (Rictor) in Alzheimer’s disease and reversal of amyloid-β expression-induced insulin resistance and toxicity in rat primary cortical neurons. J Alzheimer’s Dis 56(3):1015–1036. https://doi.org/10.3233/JAD-161029
Ya-Huang W et al (2018) Jiao-tai-wan up-regulates hypothalamic and peripheral circadian clock gene cryptochrome and activates PI3K/AKT signaling in partially sleep-deprived rats. Curr Med Sci 38(4):704–713. https://doi.org/10.1007/s11596-018-1934-x
Cao Y et al (2021) Cardioprotective effect of stem-leaf saponins from panax notoginseng on mice with sleep deprivation by inhibiting abnormal autophagy through PI3K/Akt/mTOR pathway. Front Cardiovasc Med 8(September):1–11. https://doi.org/10.3389/fcvm.2021.694219
Duan X, Pan Q, Guo L (2022) Chronic sleep deprivation impaired bone formation in growing rats and down-regulated PI3K/AKT signaling in bone tissues. Nat Sci Sleep 14:697–710. https://doi.org/10.2147/NSS.S351850
Siddiqui N, Ali J, Parvez S, Zameer S, Kalam A, Akhtar M (2021) Neuropharmacology Linagliptin, a DPP-4 inhibitor, ameliorates A β (1–42) peptides induced neurodegeneration and brain insulin resistance ( BIR ) via insulin receptor substrate-1 ( IRS-1) in rat model of Alzheimer ’ s disease. Neuropharmacology. https://doi.org/10.1016/j.neuropharm.2021.108662Received
Bajaj P, Kaur G (2022) Acute sleep deprivation - induced anxiety and disruption of hypothalamic cell survival and plasticity : a mechanistic study of protection by butanol extract of Tinospora cordifolia. Neurochem Res 47(6):1692–1706. https://doi.org/10.1007/s11064-022-03562-8
Xue R, Wan Y, Sun X, Zhang X, Gao W, Wu W (2019) Nicotinic mitigation of neuroinflammation and oxidative stress after chronic sleep deprivation. Front Immunol 10:1–12. https://doi.org/10.3389/fimmu.2019.02546
Zarneshan NS, Fakhri S, Khan H (2022) Targeting Akt_CREB_BDNF signaling pathway by ginsenosides in neurodegenerative diseases_ A mechanistic approach - ScienceDirect. Pharmacol Res 177:1
Xing C, Zhang C, Wang W, Wu L, Xing C (2020) Sleep disturbance induces increased cholesterol level by NR1D1 mediated CYP7A1 inhibition. Front Genet 11:1–10. https://doi.org/10.3389/fgene.2020.610496
Niu L et al (2021) Chronic sleep deprivation altered the expression of circadian clock genes and aggravated Alzheimer ’ s disease neuropathology. Brain Pathol. https://doi.org/10.1111/bpa.13028
Patke A, Young MW, Axelrod S (2020) Molecular mechanisms and physiological importance of circadian rhythms. Nat Rev Mol Cell Biol 21(2):67–84. https://doi.org/10.1038/s41580-019-0179-2
Zhang F et al (2020) Amyloid β redirects norepinephrine signaling to activate the pathogenic GSK3β/tau cascade. Sci Transl Med 12:526. https://doi.org/10.1126/scitranslmed.aay6931.Amyloid
Jouffe C et al (2022) Disruption of the circadian clock component BMAL1 elicits an endocrine adaption impacting on insulin sensitivity and liver disease. Proc Natl Acad Sci USA 119:10. https://doi.org/10.1073/pnas.2200083119
Ding G et al (2021) Rev-erb in GABAergic neurons controls diurnal hepatic insulin sensitivity. Nature 592(7856):763–767. https://doi.org/10.1038/s41586-021-03358-w.Rev-erb
Toledo M et al (2019) Autophagy regulates the liver clock and glucose metabolism by degrading CRY1. Cell Metab 28(2):268–281. https://doi.org/10.1016/j.cmet.2018.05.023.Autophagy
Zhao L, Liu Y, Wang X (2021) TNF-α promotes insulin resistance in obstructive sleep apnea-hypopnea syndrome. Exp Ther Med 21(6):1–7. https://doi.org/10.3892/etm.2021.10000
Singh T et al (2022) Does insufficient sleep increase the risk of developing insulin resistance: a systematic review. Cureus 14(3):1–8. https://doi.org/10.7759/cureus.23501
Mani S, Sevanan M, Krishnamoorthy A, Sekar S (2021) A systematic review of molecular approaches that link mitochondrial dysfunction and neuroinflammation in Parkinson’s disease. Neurol Sci 42(11):4459–4469. https://doi.org/10.1007/s10072-021-05551-1
Korin B, Avraham S, Azulay-debby H, Farfara D, Rolls A (2020) “Short-term sleep deprivation in mice induces B cell migration to the brain compartment”, sleep Res. Soc. https://doi.org/10.1093/sleep/zsz222
Schwarz J et al (2019) Mood impairment is stronger in young than in older adults after sleep deprivation. J. Sleep Res. 28:4. https://doi.org/10.1111/jsr.12801
Min J et al (2022) Emotion downregulation targets interoceptive brain regions while emotion upregulation targets other affective brain regions. J Neurosci 42(14):2973–2985. https://doi.org/10.1523/JNEUROSCI.1865-21.2022
De Vivo L, Bellesi M (2019) The role of sleep and wakefulness in myelin plasticity. Glia. https://doi.org/10.1002/glia.23667
Naidoo N, Ferber M, Master M, Zhu Y, Pack AI (2008) Aging impairs the unfolded protein response to sleep deprivation and leads to proapoptotic signaling. J Neurosci 28(26):6539–6548. https://doi.org/10.1523/JNEUROSCI.5685-07.2008
Wu Y, Masurat F, Preis J, Bringmann H (2018) Sleep counteracts aging phenotypes to survive starvation-induced developmental arrest in C. elegans. Curr Biol 28(22):3610-3624.e8. https://doi.org/10.1016/j.cub.2018.10.009
Naidoo N et al (2014) Aging and sleep deprivation induce the unfolded protein response in the pancreas: implications for metabolism. Aging Cell 13(1):131–141. https://doi.org/10.1111/acel.12158
Cuddapah VA, Zhang LS, Sehgal A (2019) Regulation of the blood-brain barrier by circadian rhythms and sleep. Trends 42:500–510
Palomares J et al (2015) Water exchange across the blood-brain barrier in obstructive sleep apnea: an MRI diffusion-weighted pseudo-continuous arterial spin labeling study. J. neuroimaging 25(6):900–905. https://doi.org/10.1111/jon.12288
Munoz-Ballester C, Mahmutovic D, Rafiqzad Y, Korot A, Robel S (2022) Mild traumatic brain injury-induced disruption of the blood-brain barrier triggers an atypical neuronal response. Front Cell Neurosci. https://doi.org/10.3389/fncel.2022.821885
Guo P et al (2022) Alzheimer′s disease with sleep insufficiency: a cross-sectional study on correlations among clinical characteristics, orexin, its receptors, and the blood-brain barrier. Neural Regen Res. https://doi.org/10.4103/1673-5374.360250
Festoff BW, Sajja RK, van Dreden P, Cucullo L (2016) HMGB1 and thrombin mediate the blood-brain barrier dysfunction acting as biomarkers of neuroinflammation and progression to neurodegeneration in Alzheimer’s disease. J Neuroinflammation 13(1):1–12. https://doi.org/10.1186/s12974-016-0670-z
Hurtado-Alvarado G, Velázquez-Moctezuma J, Gómez-González B (2017) Chronic sleep restriction disrupts interendothelial junctions in the hippocampus and increases blood–brain barrier permeability. J Microsc 268(1):28–38. https://doi.org/10.1111/jmi.12583
G. Artiushin, F. Li, and A. Sehgal, “Modulation of sleep by trafficking of lipids through the Drosophila blood brain barrier,” bioRxiv, p. 2022.02.17.480875, 2022, [Online]
Semyachkina-Glushkovskaya OV et al (2023) EEG biomarkers of activation of the lymphatic drainage system of the brain during sleep and opening of the blood-brain barrier. Comput Struct Biotechnol J 21:758–768. https://doi.org/10.1016/j.csbj.2022.12.019
Medina-Flores F et al (2020) Sleep loss disrupts pericyte-brain endothelial cell interactions impairing blood-brain barrier function. Brain Behav Immun 89(186):118–132. https://doi.org/10.1016/j.bbi.2020.05.077
Hurtado-Alvarado G, Domínguez-Salazar E, Velázquez-Moctezuma J, Gómez-González B (2016) A2A Adenosine Receptor Antagonism Reverts the Blood-Brain Barrier Dysfunction Induced by Sleep Restriction. PLoS ONE 11(11):1–22. https://doi.org/10.1371/journal.pone.0167236
Hurtado-Alvarado G et al (2018) The yin/yang of inflammatory status: blood-brain barrier regulation during sleep. Brain Behav Immun 69:154–166. https://doi.org/10.1016/j.bbi.2017.11.009
Sun J, Wu J, Hua F, Chen Y, Zhan F, Xu G (2020) Sleep deprivation induces cognitive impairment by increasing blood-brain barrier permeability via CD44. Front Neurol 11:1–11. https://doi.org/10.3389/fneur.2020.563916
Kaneshwaran K et al (2019) Sleep fragmentation, microglial aging, and cognitive impairment in adults with and without Alzheimer’s dementia. Sci Adv 5(12):1–13. https://doi.org/10.1126/sciadv.aax7331
Stanhope BA, Jaggard JB, Gratton M, Brown EB, Keene AC (2020) Sleep regulates glial plasticity and expression of the engulfment receptor draper following neural report sleep regulates glial plasticity and expression of the engulfment receptor draper following neural injury. Curr Biol 30(6):1092-1101.e3. https://doi.org/10.1016/j.cub.2020.02.057
Mason GM, Lokhandwala S, Riggins T, Spencer RMC, Sciences B, Program B (2021) Sleep and human cognitive development Gina. Sleep Med Rev. https://doi.org/10.1016/j.smrv.2021.101472.Sleep
Tai F et al (2020) Treadmill exercise ameliorates chronic REM sleep deprivation-induced anxiety-like behavior and cognitive impairment in C57BL/6J mice. Brain Res Bull 164:198–207. https://doi.org/10.1016/j.brainresbull.2020.08.025
Khalafi M, Alamdari K, Symonds M, Rohani H, Sakhaei M (2022) A comparison of the impact of exercise training with dietary intervention versus dietary intervention alone on insulin resistance and glucose regulation in individual with overweight or obesity: a systemic review and meta-analysis. Crit Rev Food Sci Nutr. https://doi.org/10.1080/10408398.2022.2064424
Rhea EM, Banks WA (2021) A historical perspective on the interactions of insulin at the blood-brain barrier. J Neuroendocrinol 4(33):1–19. https://doi.org/10.1111/jne.12929.A
Shokri-Kojori E et al (2018) β-Amyloid accumulation in the human brain after one night of sleep deprivation. Proc Natl Acad Sci USA 115(17):4483–4488. https://doi.org/10.1073/pnas.1721694115
Barthélemy NR, Liu H, Lu W, Kotzbauer PT, Randall J (2021) Sleep deprivation affects tau phosphorylation in human cerebrospinal fluid. Annu Neurol 87(5):700–709. https://doi.org/10.1002/ana.25702.Sleep
Li ZH, Cheng L, Wen C, Ding L, You QY, Zhang SB (2022) Activation of CNR1/PI3K/AKT Pathway by Tanshinone IIA protects hippocampal neurons and ameliorates sleep deprivation-induced cognitive dysfunction in rats. Front Pharmacol 13:1–16. https://doi.org/10.3389/fphar.2022.823732
Xie Y et al (2020) Chronic sleep fragmentation shares similar pathogenesis with neurodegenerative diseases : Endosome - autophagosome - lysosome pathway dysfunction and microglia - mediated neuroinflammation. Cns Neurosci Ther. https://doi.org/10.1111/cns.13218
Lo JC, Chong PLH, Ganesan S, Leong RLF, Chee MWL (2016) Sleep deprivation increases formation of false memory. J Sleep Res 25(6):673–682. https://doi.org/10.1111/jsr.12436
Zhang J, Yetton B, Whitehurst LN, Naji M, Mednick SC (2020) The effect of zolpidem on memory consolidation over a night of sleep. Sleep 43(11):1–12. https://doi.org/10.1093/sleep/zsaa084
Jung YJ, Oh E (2021) Is REM sleep behavior disorder a friend or foe of obstructive sleep apnea ? Clinical and etiological implications for neurodegeneration. J Clin Sleep Med. https://doi.org/10.5664/jcsm.9144REVIEW
Lucey BP et al (2021) Sleep and longitudinal cognitive performance in preclinical and early symptomatic Alzheimer ’ s disease. Brain 144:2852–2862. https://doi.org/10.1093/brain/awab272
Xu YP et al (2021) Sleep deprivation aggravates brain injury after experimental subarachnoid hemorrhage via TLR4-MyD88 pathway. Aging 13:3101–3111. https://doi.org/10.18632/aging.202503
Tartt AN, Mariani MB, Hen R, Mann JJ, Boldrini M (2022) Depression: pathogenesis and therapeutic implications. Mol Psychiatry 27(6):2689–2699. https://doi.org/10.1038/s41380-022-01520-y.Dysregulation
Tononi C (2014) Sleep and the price of plasticity: from synaptic and cellular homeostasis to memory consolidation and integration giulio. Neuron 81(1):12–34. https://doi.org/10.1016/j.neuron.2013.12.025.Sleep
Hengen KB et al (2016) Neuronal firing rate homeostasis is inhibited by sleep and promoted by wake article neuronal firing rate homeostasis is inhibited by sleep and promoted by wake. Cell 165(1):180–191. https://doi.org/10.1016/j.cell.2016.01.046
Aime M, Caicini N, Borsa M, Campelo T, Adamatidis A (2022) Paradoxical somatodendritic decoupling supports cortical plasticity during REM sleep.pdf. Neuroscience 376(6594):724–730. https://doi.org/10.1126/science.abk2734
Pignatelli M et al (2017) Synaptic plasticity onto dopamine neurons shapes article synaptic plasticity onto dopamine neurons shapes fear learning. Neuron 93(2):425–440. https://doi.org/10.1016/j.neuron.2016.12.030
Krempel R, Schleicher D, Jarvers I, Ecker A, Brunner R, Kandsperger S (2022) Sleep quality and neurohormonal and psychophysiological accompanying factors in adolescents with depressive disorders: study protocol. BJPsych Open 8(2):1–10. https://doi.org/10.1192/bjo.2022.29
Fernanez F et al (2007) Pharmacotherapy for cognitive impairment in a mouse model of down symdome. Nat Neurosci 10:411–413
Vasey C, McBride J, Penta K (2021) Circadian rhythm dysregulation and restoration: the role of melatonin. Nutrients 13(10):1–21. https://doi.org/10.3390/nu13103480
Heymann G et al (2020) Synergy of distinct dopamine projection populations in behavioral reinforcement article synergy of distinct dopamine projection populations in behavioral reinforcement. Neuron 105(5):909-920.e5. https://doi.org/10.1016/j.neuron.2019.11.024
Shi YF, Yu YQ (2013) The roles of glutamate in sleep and wakefulness. J Zhej Univ 42:583–590
Maciejczyk M, Żebrowska E, Chabowski A (2019) Insulin resistance and oxidative stress in the brain: What’s new? Int J Mol Sci 20:4. https://doi.org/10.3390/ijms20040874
Kothari V, Cardona Z, Chirakalwasan N, Anothaisintawee T, Reutrakul S (2021) Sleep interventions and glucose metabolism: systematic review and meta-analysis. Sleep Med 78:24–35. https://doi.org/10.1016/j.sleep.2020.11.035
Martin H et al (2022) Insulin modulates emotional behavior through a serotonin-dependent mechanism. Mol Psychiatry. https://doi.org/10.1038/s41380-022-01812-3
Srivastava AK, Choudhury SR, Karmakar S (2021) Neuronal Bmi-1 is critical for melatonin induced ubiquitination and proteasomal degradation of α-synuclein in experimental Parkinson’s disease models. Neuropharmacology. https://doi.org/10.1016/j.neuropharm.2020.108372
Ozansoy M et al (2020) Melatonin affects the release of exosomes and tau-content in in vitro amyloid-beta toxicity model. J Clin Neurosci 73:237–244. https://doi.org/10.1016/j.jocn.2019.11.046
Naderi-Behdani F et al (2022) Effect of melatonin on stress-induced hyperglycemia and insulin resistance in critically-ill patients: a randomized double-blind, placebo-controlled clinical trial. Casp J Intern Med 13(1):51–60. https://doi.org/10.22088/cjim.13.1.51
Guo R, Zheng H, Li Q, Qiu X, Zhang J, Cheng Z (2022) Melatonin alleviates insulin resistance through the PI3K/AKT signaling pathway in ovary granulosa cells of polycystic ovary syndrome. Reprod Biol. https://doi.org/10.1016/j.repbio.2021.100594
Shehata RSA, Mohamed-Nour ZA, Abdelrahim-Badr AM, Khalifa EM (2021) Serotonin variations and sleep disorders among shift workers. A cross-sectional study. Toxicol Ind Health 37:603–609. https://doi.org/10.1177/07482337211033135
Kim J-M et al (2022) Serotonin modifies the impact of sleep disturbance on suicidality in patients with acute coronary syndrome. Front Psychiatry 13:1–8. https://doi.org/10.3389/fpsyt.2022.1046715
Choi WG et al (2021) Inhibiting serotonin signaling through HTR2B in visceral adipose tissue improves obesity-related insulin resistance. J Clin Invest 131(23):1–12. https://doi.org/10.1172/JCI145331
Feng H et al (2020) Orexin signaling modulates synchronized excitation in the sublaterodorsal tegmental nucleus to stabilize REM sleep. Nat Commun 11:1. https://doi.org/10.1038/s41467-020-17401-3
De Sevilla MEF, Pignatelli J (2022) Insulin-like growth factor I mitigates post-traumatic stress by inhibiting AMP-kinase in orexin neurons. Mol Psychiatry 27:2182–2196. https://doi.org/10.1038/s41380-022-01442-9
Erichsen JM, Calva CB, Reagan LP, Fadel JR (2021) Physiology & Behavior Intranasal insulin and orexins to treat age-related cognitive decline. Physiol Behav 234:113370. https://doi.org/10.1016/j.physbeh.2021.113370
Green ME, Bernet V, Cheung J (2021) Thyroid dysfunction and sleep disorders. Front Endocrinol (Lausanne) 12:10–13. https://doi.org/10.3389/fendo.2021.725829
Liu PY et al (2021) Clamping cortisol and testosterone mitigates the development of insulin resistance during sleep restriction in men. J Clin Endocrinol Metab 106(9):E3436–E3448. https://doi.org/10.1210/clinem/dgab375
Rong-jin LIN, Xiao-feng DAI (2022) Sleep deprivation affects sex hormones secretion by regulating the expression of the circadian clock gene in the hypothalamus and pituitary via the PI3K / Akt signaling pathway in pregnant rats. Acta Physiol Sin 74(2019):534–540. https://doi.org/10.13294/j.aps.2022.0067
De-Oliveira RF, Da Costa-Daniele TM, Façanha CFS et al (2018) Adiponectin levels and sleep deprivation in patients with endocrine metabolic disorders. Rev Assoc Med Bras 64(12):1122–1128. https://doi.org/10.1590/1806-9282.64.12.1122
Lu M, Fang F, Wang Z, Wei P, Hu C, Wei Y (2019) Association between serum/plasma levels of adiponectin and obstructive sleep apnea hypopnea syndrome: a meta-analysis. Lipids Health Dis 18(1):1–8. https://doi.org/10.1186/s12944-019-0973-z
Brady EM et al (2018) Sleep duration, obesity and insulin resistance in a multi-ethnic UK population at high risk of diabetes. Diabetes Res Clin Pract 139:195–202. https://doi.org/10.1016/j.diabres.2018.03.010
Acknowledgements
The authors thank the Vellore Institute of Technology-Vellore for providing support and facilities.
Funding
The author does not receive any financial assistance for the article.
Author information
Authors and Affiliations
Contributions
KR Conceptualization, data collection, and writing; VDR Editing and supervision.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
This article contains no studies with human participants performed by any authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ramasubbu, K., Ramanathan, G., Venkatraman, G. et al. Sleep-associated insulin resistance promotes neurodegeneration. Mol Biol Rep 50, 8665–8681 (2023). https://doi.org/10.1007/s11033-023-08710-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-023-08710-z