Abstract
Background
MicroRNAs (miRNAs) are important nonprotein-coding genes in plants which participate in almost all biological processes during abiotic and biotic stresses. Understanding how plants respond to various environmental conditions requires the identification of stress-related miRNAs. In recent years, there has been an increased interest in studying miRNA genes and gene expression. Drought is one of the common environmental stresses limiting plant growth and development. Stress-specific miRNAs and their GRAS gene targets were validated to understand the role of miRNAs in response to osmotic stress.
Results
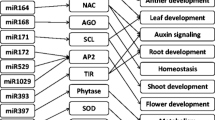
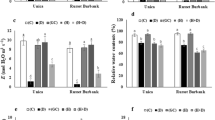
In this study, expression patterns of the ten stress-responsive miRNAs involved in osmotic stress adaptation were examined in order to undertand the regulation behavior of abiotic stress and miRNAs in two contrasting wheat genotype C-306 (drought tolerant) and WL-711 (drought sensitive). Three miRNAs were discovered to be upregulated under stress, whereas seven miRNAs were showed to be down-regulated as a consequence of the study. In contrast to miRNA, it was also discovered that GRAS genes as their targets were up-regulated during osmotic stress. In addition, the expression level of miR159, miR408 along with their targets, TaGRAS178 and TaGRAS84 increased in response to osmotic stress. Nevertheless, miR408 is highly conserved miRNA that regulates plant growth, development and stress response. As a result, variation in the expression levels of studied miRNAs in the presence of target genes provides a plausible explanation for miRNA-based abiotic stress regulation. A regulatory network of miRNA and their targets revealed that fourteen miRNA interact with 55 GRAS targets from various subfamilies that contribute in the plant growth and development.
Conclusions
These findings provide evidence for temporal and variety-specific differential regulation of miRNAs and their targets in wheat in response to osmotic shock, and they may aid in determining the potential.







Similar content being viewed by others
References
FAO (2020) Crop prospects and food situation—Quaterly Global Report
Kajla M, Yadav VK, Khokhar J et al (2015) Increase in wheat production through management of abiotic stresses: a review. J Appl Nat Sci 7:1070–1080. https://doi.org/10.31018/jans.v7i2.733
González Guzmán M, Cellini F, Fotopoulos V et al (2022) New approaches to improve crop tolerance to biotic and abiotic stresses. Physiol Plant 174:100. https://doi.org/10.1111/ppl.13547
Sallam A, Alqudah AM, Dawood MFA et al (2019) Drought stress tolerance in wheat and barley: advances in physiology, breeding and genetics research. Int J Mol Sci 20:100. https://doi.org/10.3390/ijms20133137
Samad AFA, Sajad M, Nazaruddin N et al (2017) MicroRNA and transcription factor: key players in plant regulatory network. Front Plant Sci 8:1–18. https://doi.org/10.3389/fpls.2017.00565
Boeva V (2016) Analysis of genomic sequence motifs for deciphering transcription factor binding and transcriptional regulation in eukaryotic cells. Front Genet 7:100. https://doi.org/10.3389/fgene.2016.00024
Kissoudis C, van de Wiel C, Visser RGF, van der Linden G (2014) Enhancing crop resilience to combined abiotic and biotic stress through the dissection of physiological and molecular crosstalk. Front Plant Sci 5:1–20. https://doi.org/10.3389/fpls.2014.00207
Osório J (2016) Gene regulation: Landscape and mechanisms of transcription factor cooperativity. Nat Rev Genet 17:5. https://doi.org/10.1038/nrg.2015.11
Duval I, Lachance D, Giguère I et al (2014) Large-scale screening of transcription factor-promoter interactions in spruce reveals a transcription. J Exp Bot 65:2319–2333. https://doi.org/10.1093/jxb/eru116
Dill A, Jung HS, Sun TP (2001) The DELLA motif is essential for gibberellin-induced degradation of RGA. Proc Natl Acad Sci USA 98:14162–14167. https://doi.org/10.1073/pnas.251534098
Lee S, Cheng H, King KE et al (2002) Gibberellin regulates Arabidopsis seed germination via RGL2, a GAI/RGA-like gene whose expression is up-regulated following imbibition. Genes Dev 16:646–658. https://doi.org/10.1101/gad.969002
Di Laurenzio L, Wysocka-Diller J, Malamy JE et al (1996) The SCARECROW gene regulates an asymmetric cell division that is essential for generating the radial organization of the Arabidopsis root. Cell 86:423–433. https://doi.org/10.1016/S0092-8674(00)80115-4
Li S, Zhao Y, Zhao Z et al (2016) Crystal structure of the GRAS domain of SCARECROW-LIKE7 in Oryza sativa. Plant Cell 28:1025–1034. https://doi.org/10.1105/tpc.16.00018
Bartel DP (2004) MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116:281–297. https://doi.org/10.1016/S0092-8674(04)00045-5
Murchison EP, Hannon GJ (2004) miRNAs on the move: miRNA biogenesis and the RNAi machinery. Curr Opin Cell Biol 16:223–229. https://doi.org/10.1016/j.ceb.2004.04.003
Filipowicz W, Bhattacharyya SN, Sonenberg N (2008) Mechanisms of post-transcriptional regulation by microRNAs: are the answers in sight? Nat Rev Genet 9:102–114. https://doi.org/10.1038/nrg2290
Singroha G, Sharma P, Sunkur R (2021) Current status of microRNA-mediated regulation of drought stress responses in cereals. Physiol Plant 172:1808–1821. https://doi.org/10.1111/ppl.13451
Sunkar R (2010) MicroRNAs with macro-effects on plant stress responses. Semin Cell Dev Biol 21:805–811. https://doi.org/10.1016/j.semcdb.2010.04.001
Liu H, Jin T, Liao R et al (2012) MiRFANs: an integrated database for Arabidopsis thaliana microRNA function annotations. BMC Plant Biol 12:100. https://doi.org/10.1186/1471-2229-12-68
Kim JY, Kwak KJ, Jung HJ et al (2010) MicroRNA402 affects seed germination of Arabidopsis thaliana under stress conditions via targeting DEMETER-LIKE Protein3 mRNA. Plant Cell Physiol 51:1079–1083. https://doi.org/10.1093/pcp/pcq072
Zhang HM, Kuang S, Xiong X et al (2013) Transcription factor and microRNA co-regulatory loops: important regulatory motifs in biological processes and diseases. Brief Bioinform 16:45–58. https://doi.org/10.1093/bib/bbt085
Wang H, Wang H, Shao H, Tang X (2016) Recent advances in utilizing transcription factors to improve plant abiotic stress tolerance by transgenic technology. Front Plant Sci 7:1–13. https://doi.org/10.3389/fpls.2016.00067
Zhang JW, Long Y, Xue M, De et al (2017) Identification of microRNAs in response to drought in common wild rice (Oryza rufipogon Griff.) Shoots and roots. PLoS ONE 12:1–18. https://doi.org/10.1371/journal.pone.0170330
Kozomara A, Birgaoanu M, Griffiths-Jones S (2019) MiRBase: from microRNA sequences to function. Nucleic Acids Res 47:D155–D162. https://doi.org/10.1093/nar/gky1141
Ye J, Coulouris G, Zaretskaya I et al (2012) Primer-BLAST: a tool to design target-specific primers for polymerase chain reaction. BMC Bioinform 13:134. https://doi.org/10.1186/1471-2105-13-134
Sepúlveda-García EB, Pulido-Barajas JF, Huerta-Heredia AA et al (2020) Differential expression of maize and teosinte micrornas under submergence, drought, and alternated stress. Plants 9:1–20. https://doi.org/10.3390/plants9101367
Gupta OP, Meena NL, Sharma I, Sharma P (2014) Differential regulation of microRNAs in response to osmotic, salt and cold stresses in wheat. Mol Biol Rep 41:4623–4629. https://doi.org/10.1007/s11033-014-3333-0
Barrera-Figueroa BE, Gao L, Diop NN et al (2011) Identification and comparative analysis of drought-associated microRNAs in two cowpea genotypes. BMC Plant Biol 11:100. https://doi.org/10.1186/1471-2229-11-127
Pandey B, Gupta OP, Pandey DM et al (2013) Identification of new stress-induced microRNA and their targets in wheat using computational approach. Plant Signal Behav 8:1–9. https://doi.org/10.4161/psb.23932
Kulcheski FR, Molina LG, da Fonseca GC et al (2016) Novel and conserved microRNAs in soybean floral whorls. Gene 575:213–223. https://doi.org/10.1016/j.gene.2015.08.061
Gahlaut V, Jaiswal V, Kumar A, Gupta PK (2016) Transcription factors involved in drought tolerance and their possible role in developing drought tolerant cultivars with emphasis on wheat (Triticum aestivum L). Theor Appl Genet 129:2019–2042. https://doi.org/10.1007/s00122-016-2794-z
Reyes JL, Chua NH (2007) ABA induction of miR159 controls transcript levels of two MYB factors during Arabidopsis seed germination. Plant J 49:592–606. https://doi.org/10.1111/j.1365-313X.2006.02980.x
Wang M, Wang Q, Zhang B (2013) Response of miRNAs and their targets to salt and drought stresses in cotton (Gossypium hirsutum L). Gene 530:26–32. https://doi.org/10.1016/j.gene.2013.08.009
Li J, Guo G, Guo W et al (2012) miRNA164-directed cleavage of ZmNAC1 confers lateral root development in maize (Zea mays L). BMC Plant Biol 12:1–14. https://doi.org/10.1186/1471-2229-12-220
Chen L, Wang T, Zhao M et al (2012) Identification of aluminum-responsive microRNAs in Medicago truncatula by genome-wide high-throughput sequencing. Planta 235:375–386. https://doi.org/10.1007/s00425-011-1514-9
Djami-Tchatchou AT, Sanan-Mishra N, Ntushelo K, Dubery IA (2017) Functional roles of microRNAs in agronomically important plants-potential as targets for crop improvement and protection. Front Plant Sci 8:100. https://doi.org/10.3389/fpls.2017.00378
Waheed S, Zeng L (2020) The critical role of miRNAs in regulation of flowering time and flower development. Genes (Basel) 11:1–24. https://doi.org/10.3390/genes11030319
Chen D, Yan W, Fu LY, Kaufmann K (2018) Architecture of gene regulatory networks controlling flower development in Arabidopsis thaliana. Nat Commun 9:1–13. https://doi.org/10.1038/s41467-018-06772-3
Zhou L, Liu Y, Liu Z et al (2010) Genome-wide identification and analysis of drought-responsive microRNAs in Oryza sativa. J Exp Bot 61:4157–4168. https://doi.org/10.1093/jxb/erq237
Nadarajah K, Kumar IS (2019) Drought response in rice: the miRNA story. Int J Mol Sci 20:100. https://doi.org/10.3390/ijms20153766
Mutum RD, Balyan SC, Kansal S et al (2013) Evolution of variety-specific regulatory schema for expression of osa-miR408 in indica rice varieties under drought stress. FEBS J 280:1717–1730. https://doi.org/10.1111/febs.12186
Gao Y, Feng B, Gao C et al (2022) The evolution and functional roles of miR408 andits targets in plants. Int J Mol Sci 23:6–10. https://doi.org/10.3390/ijms23010530
Kouhi F, Sorkheh K, Ercisli S (2020) MicroRNA expression patterns unveil differential expression of conserved miRNAs and target genes against abiotic stress in safflower. PLoS ONE 15:1–18. https://doi.org/10.1371/journal.pone.0228850
Feng XM, Qiao Y, Mao K et al (2010) Overexpression of Arabidopsis AtmiR408 gene in tobacco. Acta Biol Cracoviensia Ser Bot 52:26–31. https://doi.org/10.2478/v10182-010-0020-x
Zhang F, Yang J, Zhang N et al (2022) Roles of microRNAs in abiotic stress response and characteristics regulation of plant. Front Plant Sci 13:100. https://doi.org/10.3389/fpls.2022.919243
Engstrom EM, Andersen CM, Gumulak-Smith J et al (2011) Arabidopsis homologs of the petunia HAIRY MERISTEM gene are required for maintenance of shoot and root indeterminacy. Plant Physiol 155:735–750. https://doi.org/10.1104/pp.110.168757
Acknowledgements
We would like to thank ICAR-IIWBR for providing the facility for the conductance of this experiment. This paper is IIWBR contribution number 82/479.
Funding
This study was supported by Indian Council of Agricultural Research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mishra, S., Chaudhary, R. & Sharma, P. Temporal expression analysis of microRNAs and their target GRAS genes induced by osmotic stress in two contrasting wheat genotypes. Mol Biol Rep 50, 5621–5633 (2023). https://doi.org/10.1007/s11033-023-08486-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-023-08486-2