Abstract
Background
The PLAUR gene encodes the urokinase-like plasminogen activator receptor (uPAR) and may undergo alternative splicing. Excluding cassette exons 3, 5 and 6 from the transcript results in truncated protein variants whose precise functions have not been elucidated yet. The PLAUR gene is one of several expressed in myeloid cells, where uPAR participates in different cellular processes, including the contact activation system and kallikrein-kinin system, which play an important role in hereditary angioedema (HAE) pathogenesis. A hypothesis about the PLAUR splicing pattern impact on HAE severity was tested.
Methods and results
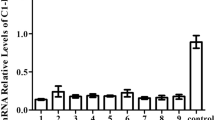
The RT-PCR quantified by capillary electrophoresis was used. Although no significant difference in alternative transcript frequency was observed between healthy volunteers and HAE patients, a significant increase in all cassette exon inclusion variants was revealed during monocyte-to-macrophage differentiation.
Conclusions
PLAUR alternative splicing in monocytes and macrophages neither was different between HAE patients and healthy controls, nor reflected disease severity. However, the results showed an PLAUR splicing pattern was changing during monocyte-to-macrophage differentiation, but the significance of these changes is unknown and awaits future clarification.



Similar content being viewed by others
Data availability
Manuscript has data included as supplementary material.
References
Pyke C, Eriksen J, Solberg H et al (1993) An alternatively spliced variant of mRNA for the human receptor for urokinase plasminogen activator. FEBS Lett 326:69–74. https://doi.org/10.1016/0014-5793(93)81763-P
Lv T, Zhao Y, Jiang X et al (2021) UPAR: an essential factor for tumor development. J Cancer 12:7026–7040. https://doi.org/10.7150/jca.62281
Smith HW, Marshall CJ (2010) Regulation of cell signalling by uPAR. Nat Rev Mol Cell Biol 11:23–36. https://doi.org/10.1038/nrm2821
Kjaergaard M (2008) Structure and ligand interactions of the urokinase receptor (uPAR). Front Biosci 13:5441–5461. https://doi.org/10.2741/3092
Degryse B, Resnati M, Czekay R-P et al (2005) Domain 2 of the Urokinase receptor contains an integrin-interacting epitope with intrinsic signaling activity. J Biol Chem 280:24792–24803. https://doi.org/10.1074/jbc.M413954200
Ferraris GMS, Schulte C, Buttiglione V et al (2014) The interaction between uPAR and vitronectin triggers ligand-independent adhesion signalling by integrins. EMBO J 33:2458–2472. https://doi.org/10.15252/embj.201387611
Franco P, Vocca I, Carriero MV et al (2006) Activation of urokinase receptor by a novel interaction between the connecting peptide region of urokinase and αvβ5 integrin. J Cell Sci 119:3424–3434. https://doi.org/10.1242/jcs.03067
Dumler I, Weis A, Mayboroda OA et al (1998) The Jak/Stat pathway and Urokinase receptor signaling in human aortic vascular smooth muscle cells. J Biol Chem 273:315–321. https://doi.org/10.1074/jbc.273.1.315
Liu Y, Pan YF, Xue Y et al (2018) uPAR promotes tumor-like biologic behaviors of fibroblast-like synoviocytes through PI3K/Akt signaling pathway in patients with rheumatoid arthritis. Cell Mol Immunol 15:171–181. https://doi.org/10.1038/cmi.2016.60
Breuss JM, Uhrin P (2012) VEGF-initiated angiogenesis and the uPA/uPAR system. Cell Adh Migr 6:535–615. https://doi.org/10.4161/cam.22243
Stewart CE, Sayers I (2009) Characterisation of urokinase plasminogen activator receptor variants in human airway and peripheral cells. BMC Mol Biol 10:75. https://doi.org/10.1186/1471-2199-10-75
Mahdi F, Shariat-Madar Z, Kuo A et al (2004) Mapping the interaction between high molecular mass Kininogen and the Urokinase Plasminogen activator receptor. J Biol Chem 279:16621–16628. https://doi.org/10.1074/jbc.M313850200
Li Y, Lawrence DA, Zhang L (2003) Sequences within domain II of the Urokinase receptor critical for differential ligand recognition. Journal of Biological Chemistryv 278:29925–29932. https://doi.org/10.1074/jbc.M300751200
Bdeir K, Kuo A, Mazar A et al (2000) A region in domain II of the Urokinase receptor required for Urokinase binding. J Biol Chem 275:28532–28538. https://doi.org/10.1074/jbc.M001595200
Mazar AP (2008) Urokinase plasminogen activator receptor choreographs multiple ligand interactions: implications for tumor progression and therapy. Clin Cancer Res 14:5649–5655. https://doi.org/10.1158/1078-0432.CCR-07-4863
Leth JM, PlougM, (2021) Targeting the Urokinase-type plasminogen activator Receptor (uPAR) in human diseases with a view to non-invasive imaging and therapeutic intervention. Front Cell Develop Biol. https://doi.org/10.3389/fcell.2021.732015
Stewart CE, Nijmeh HS, Brightling CE et al (2012) UPAR regulates bronchial epithelial repair in vitro and is elevated in asthmatic epithelium. Thorax 67:477–487. https://doi.org/10.1136/thoraxjnl-2011-200508
Bindke G, Gehring M, Wieczorek D et al (2022) Identification of novel biomarkers to distinguish bradykinin-mediated angioedema from mast cell-/histamine-mediated angioedema. Allergy 77:946–955. https://doi.org/10.1111/all.15013
Castellano G, Divella C, Sallustio F et al (2018) A transcriptomics study of hereditary angioedema attacks. J Allergy Clin Immunol 142:883–891. https://doi.org/10.1016/j.jaci.2018.03.016
Mahdi F, Shariat-Madar Z, Todd RF et al (2001) Expression and colocalization of cytokeratin 1 and urokinase plasminogen activator receptor on endothelial cells. Blood 97:2342–2350. https://doi.org/10.1182/blood.V97.8.2342
Mahdi F, Shariat-Madar Z, Figueroa CD et al (2002) Factor XII interacts with the multiprotein assembly of urokinase plasminogen activator receptor, gC1qR, and cytokeratin 1 on endothelial cell membranes. Blood 99:3585–3596. https://doi.org/10.1182/blood.V99.10.3585
Shariat-Madar Z, Mahdi F, Schmaier AH (2002) Assembly and activation of the plasma kallikrein/kinin system: a new interpretation. Int Immunopharmacol 2:1841–1849. https://doi.org/10.1016/S1567-5769(02)00178-9
Schmaier AH (2016) The contact activation and kallikrein/kinin systems: pathophysiologic and physiologic activities. J Thromb Haemost 14:28–39. https://doi.org/10.1111/jth.13194
Kanse SM, Chavakis T, Al-Fakhri N et al (2004) Reciprocal Regulation of Urokinase Receptor (CD87)-mediated Cell Adhesion by Plasminogen Activator inhibitor-1 and Protease nexin-1. J Cell Sci 117:477–485. https://doi.org/10.1242/jcs.00861
Dong C, Zhao Z, M, et al (2013) RNA sequencing and transcriptomal analysis of human monocyte to macrophage differentiation. Gene 519:279–287. https://doi.org/10.1016/j.gene.2013.02.015
Luther T, Kotzsch M, Meye A et al (2003) Identification of a novel urokinase receptor splice variant and its prognostic relevance in breast cancer. Thromb Haemost 89:705–717. https://doi.org/10.1055/s-0037-1613577
Caiolfa VR, Zamai M, Malengo G et al (2007) Monomer–dimer dynamics and distribution of GPI-anchored uPAR are determined by cell surface protein assemblies. J Cell Biol 179:1067–1082. https://doi.org/10.1083/jcb.200702151
Madsen CD, Ferraris GMS, Andolfo A et al (2007) UPAR-induced cell adhesion and migration: vitronectin provides the key. J Cell Biol 177:927–939. https://doi.org/10.1083/jcb.200612058
Ferraris GMS, Schulte C, Buttiglione V et al (2014) The interaction between uPAR and vitronectin triggers ligand‐independent adhesion signalling by integrins. The EMBO Journal 33:2458–2472. https://doi.org/10.15252/embj.201387611
Plesner T, Behrendt N, Ploug M (1997) Structure, function and expression on blood and bone marrow cells of the Urokinase-Type Plasminogen Activator Receptor, uPAR. Stem Cells 15:398–408. https://doi.org/10.1002/stem.150398
Reuning U, Sperl S, Kopitz C et al (2003) Urokinase-type Plasminogen Activator (uPA) and its Receptor (uPAR): Development of Antagonists of uPA / uPAR Interaction and their Effects In Vitro and In Vivo. Curr Pharm Des 9:1529–1543. https://doi.org/10.2174/1381612033454612
Larusch GA, Mahdi F, Shariat-Madar Z et al (2010) Factor XII stimulates ERK1/2 and Akt through uPAR, integrins, and the EGFR to initiate angiogenesis. Blood 115:5111–5120. https://doi.org/10.1182/blood-2009-08-236430
Andreasen PA, Kjøller L, Christensen L et al (1998) The urokinase-type plasminogen activator system in cancer metastasis: a review. Int J Cancer 72:1–22. https://doi.org/10.1002/(SICI)1097-0215(19970703)72:13.0.CO;2-Z
Kaplan AP, Austen KF (1971) A prealbumin activator of prekallikrein. II. Derivation of activators of prekallikrein from active Hageman factor by digestion with plasmin. J Exp Med 133:696–712. https://doi.org/10.1084/jem.133.4.696
Larusch GA, Merkulova A, Mahdi F et al (2013) Domain 2 of uPAR regulates single-chain urokinase-mediated angiogenesis through β 1 -integrin and VEGFR2. Am J Physiol-Heart Circulatory Physiol 305:305–320. https://doi.org/10.1152/ajpheart.00110.2013
Gyetko MR, Todd RF, Wilkinson CC et al (1994) The urokinase receptor is required for human monocyte chemotaxis in vitro. J Clin Investig 93:1380–1387. https://doi.org/10.1172/JCI117114
Liu H, Lorenzini PA, Zhang F et al (2018) Alternative splicing analysis in human monocytes and macrophages reveals MBNL1 as major regulator. Nucleic Acids Res 46:6069–6086. https://doi.org/10.1093/nar/gky401
Nusrat AR, Chapman HA (1991) An autocrine role for urokinase in phorbol ester-mediated differentiation of myeloid cell lines. J Clin Investig 87:1091–1097. https://doi.org/10.1172/JCI115070
Paland N, Aharoni S, Fuhrman B (2013) Urokinase-type plasminogen activator (uPA) modulates monocyte-to-macrophage differentiation and prevents Ox-LDL-induced macrophage apoptosis. Atherosclerosis 231:29–38. https://doi.org/10.1016/j.atherosclerosis.2013.08.016
Yang A, Dai J, Xie Z et al (2014) High molecular weight kininogen binds phosphatidylserine and opsonizes Urokinase Plasminogen Activator Receptor-mediated efferocytosis. J Immunol 192:4398–4408. https://doi.org/10.4049/jimmunol.1302590
Khan MM, Bradford HN, Isordia-Salas I et al (2006) High-Molecular-Weight Kininogen Fragments Stimulate the Secretion of Cytokines and Chemokines through uPAR, Mac-1, and gC1qR in monocytes. Arterioscler Thromb Vasc Biol 26:2260–2266. https://doi.org/10.1161/01.ATV.0000240290.70852.c0
Arcoleo F, Lo Pizzo M, Misiano G et al (2018) The complex alteration in the network of IL-17-type cytokines in patients with hereditary angioedema. Clin Exp Med 18:355–361. https://doi.org/10.1007/s10238-018-0499-0
GrymovaT VM, Soucek P et al (2019) Neutrophils are dysregulated in patients with hereditary angioedema types I and II in a symptom-free period. Mediators Inflamm 2019:9515628. https://doi.org/10.1155/2019/9515628
Funding
The study was supported by grant numbers NV18-05–00330 and NU21-05–00438 from the Ministry of Health of the Czech Republic, and Specific University Research Grant number MUNI/A/1244/2021 provided by the Ministry of Education, Youth and Sports of the Czech Republic.
Author information
Authors and Affiliations
Contributions
Přemysl Souček, Tomáš Freiberger, Marcela Vlková and Jiří Litzman contributed to the study conception and design. Material preparation, data collection and analysis were performed by Lucie Ballonová, Peter Slanina, Julie Štíchová, Roman Hakl and Přemysl Souček. The first draft of the manuscript was written by Petra Kulíšková and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Ethical approval
The study was approved by the Medical Ethics Committee of St. Anne’s University Hospital (ethics approval number: 6G/2015, Brno). Informed consent was obtained from all the participants before being included in the study. The study conforms to the Declaration of Helsinki standards.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ballonová, L., Kulíšková, P., Slanina, P. et al. PLAUR splicing pattern in hereditary angioedema patients’ monocytes and macrophages. Mol Biol Rep 50, 4975–4982 (2023). https://doi.org/10.1007/s11033-023-08391-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-023-08391-8