Abstract
Background
Turkey is not only a center of origin for wheat, but also contains wild forms of various cereals. Turkey, located in the Fertile Crescent, has conserved its genetic richness to the present day. The aim of the study was to investigate the genetic diversity of 70 wild wheat species, to evaluate the structure of diversity in germplasm and to generate useful data for further breeding programs.
Methods and results
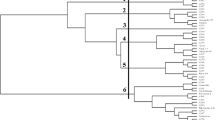
Genetic diversity and population structure of 70 wild wheat species (Ae. cylindrica, Ae. geniculata, Ae. triuncialis, T. dicocoides, Ae. columnaris) collected from Eastern and Southeastern Anatolia regions of Turkey were investigated in this study with the use of inter-primer binding site (iPBS) markers. Of 35 iPBS primers used, 11 yielded a total of 61 alleles. Number of alleles per marker varied between 2 (iPBS-2085) and 9 (iPBS-2394) with an average value of 5.55. Polymorphic information content (PIC) values varied between 0.22 and 0.47, with an average value of 0.35. Average number of effective alleles (Ne) was identified as 1.9488, Nei’s genetic diversity (H) as 0,4861 and Shannon’s information index (I) as 0.6791. Cluster analysis through unweighted pair-group mean average (UPGMA) method revealed that 70 wild wheats were divided into three main clusters. Genetic similarity between the genotypes, calculated with the use of NTSYS-pc software, varied between 19% (YB2 and YB70) and 98% (YB66 and YB67). Principal coordinate analysis (PCoA) revealed that three principal coordinates explained 62.33% of total variation. Moreover, population structure analysis showed that all genotypes formed three sub-populations. Expected heterozygosity values varied between 0.2666 (the first sub-population) and 0.2330 (third sub-population), with an average value of 0.2500. Average population differentiation measurement (Fst) was identified as 0.3716 for the first sub-population, 0.3930 for the second subpopulation and 0.4804 for the third sub-population.
Conclusions
Based on present findings population structure of 70 wild wheat genotypes collected from Eastern and Southeastern Anatolia regions of Turkey were successfully characterized with the use of iPBS markers. Present findings suggested that iPBS-retrotransposon markers could reliably be used to elucidate genetic diversity of Aegilops genotypes.



Similar content being viewed by others
References
Shiferaw B, Smale M, Braun H-J, Duveiller E, Reynolds M, Muricho G (2013) Crops that feed the world 10. Past successes and future challenges to the role played by wheat in global food security. Food Secur 5(3):291–317. https://doi.org/10.1007/s12571-013-0263-y
Sharma S, Schulthess AW, Bassi FM, Badaeva ED, Neumann K, Graner A, Özkan H, Werner P, Knüpffer H, Kilian B (2021) Introducing beneficial alleles from plant genetic resources into the wheat germplasm Biology. 10:982. https://doi.org/10.3390/biology10100982. 10
Hammer K, Gladis T (2014) Notes on infraspecific nomenclature and classifications of cultivated plants in Compositae, Cruciferae, Cucurbitaceae, Gramineae (with a remark on Triticum dicoccon Schrank) and Leguminosae. Genet Resour Crop Evol 61(8):1455–1467. https://doi.org/10.1007/s10722-014-0148-8
Dorofeev V, Filatenko A, Migushova E, Udachin R, Jakubziner M (1979) Wheat. In: Dorofeev VF, Korovina ON (eds) Flora of cultivated plants, vol 1. Kolos, Leningrad. (in Russian)
Zeibig F, Kilian B, Frei M (2021) The grain quality of wheat wild relatives in the evolutionary context. Theor Appl Genet 1–20. https://doi.org/10.1007/s00122-021-04013-8
Matsuoka Y (2011) Evolution of polyploid Triticum wheats under cultivation: the role of domestication, natural hybridization and allopolyploid speciation in their diversification. Plant Cell Physiol 52(5):750–764. https://doi.org/10.1093/pcp/pcr018
Kumar A, Sharma A, Sharma R, Srivastva P, Choudhary A (2021) Exploration of wheat wild relative diversity from Lahaul valley: a cold arid desert of Indian Himalayas. Cereal Res Commun 1–16. https://doi.org/10.1007/s42976-021-00166-w
Leśniowska-Nowak J, Okoń S, Wieremczuk A (2021) Molecular diversity analysis of genotypes from four Aegilops species based on retrotransposon–microsatellite amplified polymorphism (REMAP) markers. Cereal Res Commun 49(1):37–44. https://doi.org/10.1007/s42976-020-00086-1
Iizumi T, Ali-Babiker I-EA, Tsubo M, Tahir IS, Kurosaki Y, Kim W, Gorafi YS, Idris AA, Tsujimoto H (2021) Rising temperatures and increasing demand challenge wheat supply in Sudan. Nat Food 2(1):19–27. https://doi.org/10.1038/s43016-020-00214-4
Hegde S, Valkoun J, Waines J (2002) Genetic diversity in wild and weedy Aegilops, Amblyopyrum, and Secale species—a preliminary survey. Crop Sci 42(2):608–614. https://doi.org/10.2135/cropsci2002.6080
Arzani A, Ashraf M (2016) Smart engineering of genetic resources for enhanced salinity tolerance in crop plants. CRC Crit Rev Plant Sci 35(3):146–189. https://doi.org/10.1080/07352689.2016.1245056
Kilian B, Dempewolf H, Guarino L, Werner P, Coyne C, Warburton ML (2021) Crop Science special issue: Adapting agriculture to climate change: A walk on the wild side. Crop Sci 61(1):32–36. https://doi.org/10.1002/csc2.20418
Henry RJ, Nevo E (2014) Exploring natural selection to guide breeding for agriculture. Plant Biotechnol J 12(6):655–662. https://doi.org/10.1111/pbi.12215
Li M, Dong L, Li B, Wang Z, Xie J, Qiu D, Li Y, Shi W, Yang L, Wu Q (2020) A CNL protein in wild emmer wheat confers powdery mildew resistance. New Phytol 228(3):1027–1037. https://doi.org/10.1111/nph.16761
Dvorak J (1998) Genome analysis in the Triticum-Aegilops alliance. In: Proceedings of the 9th international wheat genetics symposium: Saskatoon: 8–11
Mohammadi SA, Prasanna B (2003) Analysis of genetic diversity in crop plants—salient statistical tools and considerations. Crop sci 43(4):1235–1248. https://doi.org/10.2135/cropsci2003.1235
Pour-Aboughadareh A, Ahmadi J, Mehrabi A, Etminan A, Moghaddam M (2017) Assessment of genetic diversity among Iranian Triticum germplasm using agro-morphological traits and start codon targeted (SCoT) markers. Cereal Res Commun 45(4):574–586. https://doi.org/10.1556/0806.45.2017.033
Mguis K, Mahjoub A, Abassi M, Albouchi A, Ouerghi Z, Nadia BB, Béjaoui Z (2015) Morphological and genetic variation in Aegilops geniculata Roth. from Tunisia. Int J Agric Res 6:8–21
Zaharieva M, Gaulin E, Havaux M, Acevedo E, Monneveux P (2001) Drought and heat responses in the wild wheat relative Aegilops geniculata Roth: potential interest for wheat improvement. Crop Sci 41(4):1321–1329. https://doi.org/10.2135/cropsci2001.4141321x
Goryunova S, Chikida N, Kochieva E (2017) AFLP, RAPD, and ISSR analysis of intraspecific polymorphism and interspecific differences of allotetraploid species Aegilops kotschyi Boiss. and Aegilops variabilis. Eig Russ J Genet 53(5):568–575. https://doi.org/10.1134/S1022795417050040
Farouji AE, Khodayari H, Saeidi H, Rahiminejad MR (2015) Genetic diversity of diploid Triticum species in Iran assessed using inter-retroelement amplified polymorphisms (IRAP) markers. Biologia 70(1):52–60. https://doi.org/10.1515/biolog-2015-0002
Taheri MT, Alavi-Kia SS, Mohammadi SA, Vahed MM (2018) Assessment of genetic diversity and relationships among Triticum urartu and Triticum boeoticum populations from Iran using IRAP and REMAP markers. Genet Resour Crop Evol 65(7):1867–1878. https://doi.org/10.1007/s10722-018-0660-3
Ahmadi J, Fabriki OS, Pour AA (2019) Evaluation of genetic diversity in Aegilops populations possessing D genome using SCoT and TRAP markers. MGJ 14(3):2019–2228
Bouziani MC, Bechkri S, Bellil I, Khelifi D (2019) Evaluation of genetic diversity of Algerian Aegilops ventricosa Tausch. using inter-simple sequence repeat (ISSR) markers. World j Environ Biosci 8:1–6
Abbasov M, Brueggeman R, Raupp J, Akparov Z, Aminov N, Bedoshvili D, Gross T, Gross P, Babayeva S, Izzatullayeva V (2019) Genetic diversity of Aegilops L. species from Azerbaijan and Georgia using SSR markers. Genet Resour Crop Evol 66(2):453–463. https://doi.org/10.1007/s10722-018-0725-3
Yamane K, Kawahara T (2018) Size homoplasy and mutational behavior of chloroplast simple sequence repeats (cpSSRs) inferred from intra-and interspecific variations in four chloroplast regions of diploid and polyploid Triticum and Aegilops species. Genet Resour Crop Evol 65(3):727–743. https://doi.org/10.1007/s10722-017-0567-4
Gong W, Han R, Li H, Song J, Yan H, Li G, Liu A, Cao X, Guo J, Zhai S (2017) Agronomic traits and molecular marker identification of wheat–Aegilops caudata addition lines. Front Plant Sci 8:1743. https://doi.org/10.3389/fpls.2017.01743
Urazaliev R, Yessimbekova M, Mukin K, Chirkin A, Ismagulova G (2018) Monitoring of Aegilops L local species genetic diversity of Kazakhstans flora Vavilovskii Zhurnal Genetikii Selektsii Vavilov. J Genet Plant Breed 22:484–490. https://doi.org/10.18699/VJ18.386
Yasui Y, Nasuda S, Matsuoka Y, Kawahara T (2001) The Au family, a novel short interspersed element (SINE) from Aegilops umbellulata. Theor Appl Genet 102(4):463–470
Pour-Aboughadareh A, Etminan A, Shooshtari L, Maleki-Tabrizi N (2020) Comparative assessment of SCoT and CBDP markers for investigation of genetic diversity existing in different aegilops species. World Res J Agric Biotechnol 11(4):153–174. https://doi.org/10.22103/JAB.2020.2528
Abbasov M, Sansaloni CP, Burgueño J, Petroli CD, Akparov Z, Aminov N, Babayeva S, Izzatullayeva V, Hajiyev E, Rustamov K (2020) Genetic diversity analysis using DArTseq and SNP markers in populations of Aegilops species from Azerbaijan. Genet Resour Crop Evol 67(2):281–291. https://doi.org/10.1007/s10722-019-00866-7
Su Y, Zou M, Zhu Y, Han X, Li Y, Zhang D, Li S (2020) Analysis of population structure and origin in Aegilops tauschii Coss. from China through SNP markers. Genet Resour Crop Evol 67(4):923–934. https://doi.org/10.1007/s10722-020-00890-y
Hosseinpour A, Haliloglu K, Ozkan G, Tan M (2019) Genetic diversity and population structure of quinoa (Chenopodium quinoa Willd.) using iPBS-retrotransposons markers. Appl Ecol Environ Res 17(2):1899–1911. https://doi.org/10.15666/aeer/1702_18991911
Hosseinpour A, Karahan F, İlhan E, İlçim A, Haliloğlu K (2019) Genetic structure and diversity of Adonis L. (Ranunculaceae) populations collected from Turkey by inter-primer binding site (iPBS) retrotransposon markers. Turk J Bot 43(5):585–596. https://doi.org/10.3906/bot-1810-1
Kole C (2011) Wild crop relatives: genomics and breeding resources: plantation and ornamental crops. Springer
Zeinalzadehtabrizi H, Hosseinpour A, Aydin M, Haliloglu K (2015) A modified genomic DNA extraction method from leaves of sunflower for PCR based analyzes. J Bio Env Sci 7(6):222–225
Kalendar R, Antonius K, Smýkal P, Schulman AH (2010) iPBS: a universal method for DNA fingerprinting and retrotransposon isolation. Theor Appl Genet 121(8):1419–1430. https://doi.org/10.1007/s00122-010-1398-2
Dice LR (1945) Measures of the amount of ecologic association between species. Ecology 26(3):297–302. https://doi.org/10.2307/1932409
Rohlf FJ (1972) An empirical comparison of three ordination techniques in numerical taxonomy. Syst Zool 21(3):271–280. https://doi.org/10.1093/sysbio/21.3.271
Peakall R, Smouse PE (2006) GENALEX 6: genetic analysis in Excel. Population genetic software for teaching and research. Mol Ecol Notes 6(1):288–295. https://doi.org/10.1111/j.1471-8286.2005.01155.x
Anderson JA, Churchill G, Autrique J, Tanksley S, Sorrells M (1993) Optimizing parental selection for genetic linkage maps. Genome 36(1):181–186. https://doi.org/10.1139/g93-024
Yeh FC, Yang R, Boyle TB, Ye Z, Mao JX (1997) POPGENE, the user-friendly shareware for population genetic analysis. Molecular biology and biotechnology center. Univ Alta Can 10:295–301
Pritchard JK, Stephens M, Rosenberg NA, Donnelly P (2000) Association mapping in structured populations. Am J Hum Genet 67(1):170–181. https://doi.org/10.1086/302959
Evanno G, Regnaut S, Goudet J (2005) Detecting the number of clusters of individuals using the software STRUCTURE: a simulation study. Mol Ecol 14(8):2611–2620. https://doi.org/10.1111/j.1365-294X.2005.02553.x
Earl DA, VonHoldt BM (2012) STRUCTURE HARVESTER: a website and program for visualizing STRUCTURE output and implementing the Evanno method. Conserv Genet Res 4(2):359–361. https://doi.10.1007/s12686-011-9548-7
Nadeem MA (2021) Deciphering the genetic diversity and population structure of Turkish bread wheat germplasm using iPBS-retrotransposons markers. Mol Biol Rep 48(10):6739–6748. https://doi.org/10.1007/s11033-021-06670-w
Öztürk H, Dursun A, Hosseinpour A, Haliloğlu K (2020) Genetic diversity of pinto and fresh bean (Phaseolus vulgaris L.) germplasm collected from Erzincan province of Turkey by inter-primer binding site (iPBS) retrotransposon markers. Turk J Agric For 44(4):417–427. https://doi.org/10.3906/tar-2002-9
Karagoz H, Cakmakci R, Hosseinpour A, Ozkan G, Haliloglu K (2020) Analysis of genetic variation and population structure among of oregano (Origanum acutidens L.) accessions revealed by agro-morphological traits, oil constituents and retrotransposon-based inter-primer binding sites (iPBS) markers. Genet Resour Crop Evol 67(6):1367–1384. https://doi.org/10.1007/s10722-020-00887-7
Ghobadi G, Etminan A, Mehrabi AM, Shooshtari L (2021) Molecular diversity analysis in hexaploid wheat (Triticum aestivum L.) and two Aegilops species (Aegilops crassa and Aegilops cylindrica) using CBDP and SCoT markers. J Genet Eng Biotechnol 19(1):1–11. https://doi.org/10.1186/s43141-021-00157-8
Laity T, Laffan SW, González-Orozco CE, Faith DP, Rosauer DF, Byrne M, Miller JT, Crayn D, Costion C, Moritz CC (2015) Phylodiversity to inform conservation policy: An Australian example. Sci Total Environ 534:131–143. https://doi.org/10.1016/j.scitotenv.2015.04.113
Funding
This research was financially supported by Scientific Research Projects Department of Mardin Artuklu University (Grant Number: MAU.BAP.20.KMY.024).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Authors declare no conflict of interest.
Ethical approval:
This manuscript does not contain any studies with human participants or animals performed by any of the authors.
Consent to participate and publish
All authors reviewed and approved the final version for publication.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kizilgeci, F., Bayhan, B., Türkoğlu, A. et al. Exploring genetic diversity and Population structure of five Aegilops species with inter-primer binding site (iPBS) markers. Mol Biol Rep 49, 8567–8574 (2022). https://doi.org/10.1007/s11033-022-07689-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-022-07689-3