Abstract
The world is grappling with an unprecedented public health crisis COVID-19 pandemic caused by the novel coronavirus SARS-CoV-2. Due to the high transmission/mortality rates and socioeconomic impacts of the COVID-19, its control is crucial. In the absence of specific treatment, vaccines represent the most efficient way to control and stop the pandemic. Many companies around the world are currently making efforts to develop the vaccine to combat COVID-19. This review outlines key strategies for generating SARS-CoV-2 vaccine candidates, along with the mechanism of action, advantages, and potential limitations of each vaccine. The use of nanomaterials and nanotechnology for COVID-19 vaccines development will also be discussed.
Similar content being viewed by others
Introduction
From the beginning of the coronavirus disease (COVID-19), the world faced a major health crisis. After months of fighting the disease, medical supplies are often scarce, hospitals are overwhelmed, and medical staff are exhausted. The high prevalence and severe clinical impact of COVID-19 have forced governments around the world to take strict measures, including quarantine, social distancing, and isolation of patients with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, to prevent the spread of the virus and to tackle pandemic [1]. However, the incidence and mortality rate of COVID-19 is still high, especially because of the constant mutations in the viral structure of SARS-CoV-2. The rate of mortality and severity of COVID-19 is higher in elderly persons, and patients with immunodeficiency diseases, cancer, diabetes, and cardiovascular disease [2]. As of 20th December 2021, COVID-19 has already caused 273,900,334 confirmed cases globally, including 5,351,812 deaths, reported to WHO [3]. Given the urgent need to manage and treat COVID-19 patients, a variety of treatment options from antiviral agents, repurposed drugs, and protease inhibitors to convalescent plasma and monoclonal antibodies are in clinical trials [4]. Nevertheless, no licensed and effective treatment for this disease has been found. In the challenging situation of the COVID-19 pandemic and the lack of approved therapeutic protocol, it seems vaccination can be the best approach to controlling the disease. Generally, vaccination is one of the most effective public health interventions that modern medicine offers to prevent the large-scale spread of infectious diseases economically [5]. The impact of scientific advances is quite evident in COVID-19 vaccine development, as in this case the common required time for the effective vaccine has been reduced from more than a decade to about a year. However, leverage from what has been learned from prior infectious disease outbreaks; severe acute respiratory syndrome (SARS) and the Middle East respiratory syndrome (MERS), along with research from recent years characterizing next-generation and high-tech vaccine platforms helped to accelerate the process and shorten the development timeline [6].
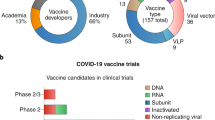
According to last data from WHO on December 17, 2021, 137 vaccine candidates are undergoing human clinical trials worldwide and 194 vaccine candidates are in the preclinical development stage. Of these, at least six COVID-19 vaccine trials have reported very promising provisional results and a total of 8,387,658,165 vaccine doses have been administered [7]. In this context, a variety of platform technologies have been employed by various research groups to make notable advances in developing vaccines in a short time. These vaccine platforms are ranging from virus-based (live attenuated and inactivated) and protein-based (subunit and virus-like particle) to novel gene-based and nano-based strategies. In this review, the principles and design considerations in COVID-19 vaccine development, including antigen selection, the routes and regimens of vaccination, and various vaccine platforms are discussed. We address the importance of nanotechnology to design COVID-19 vaccine candidates. Finally, we discuss advantages, disadvantages, and challenges regarding each COVID-19 vaccine platform.
COVID-19 vaccine development
Viral vaccines are biological products that can induce activity. Because vaccination is probably the most effective way to overcome the pandemic, attempts to develop safe and effective SARS-CoV-2 vaccines have been ignited in several countries. Before using a vaccine in a larger population, its safety and efficacy must be fully determined. Ineffective vaccines not only cannot protect against the virus but can also cause diseases through antibody-dependent boosting or other mechanisms [8]. Before vaccine development, the collection of important information, including target antigen detection, immunization pathway, vaccine platform, correlated-immune protection, animal models, scalability, production capability, outbreak estimating, and target population must be clearly defined [9]. To date, more than 200 candidates for the COVID-19 vaccine are being developed in various stages of trials and the number is continuing to grow [10]. This diversity of vaccine candidates faces a wide range of challenges from producing, manufacturing, storing to injecting them into vulnerable individuals in the short term. Choosing the right type of vaccine, carrier or vector, adjuvant, excipients, dosage form, and route of administration can be a solution to overcome these challenges and also have a direct impact on the effectiveness of the vaccine against COVID-19 [10].
In traditional (classical) vaccines, the entry of a dead or weakened pathogen into a healthy person's body warns his/her immune system that some foreign organisms have invaded the body and must be removed [11]. In the classic platform, antigens derived from inactive or semi-active bacteria or weak viruses can cause disease once they enter the body, but are still able to activate the immune system to make its cells produce antibodies. Since then, if a person comes in contact with a native pathogen, the body's immune system prepares the necessary antibodies and multiplies them much faster because it has already been sensitized to the vaccine [11].
Similar to what has already been described for therapeutic development, the remarkable genomic match of SARS-CoV-2 to different coronaviruses helps vaccine developers to design the most promising candidates for the COVID-19 vaccine [12]. Artificial intelligence and other computational tools are currently being used to accelerate the process of vaccine development against this new pathogen [12, 13]. Moreover, in order to minimize the viable dose and to expand the therapeutic and safety window, the enhancement of immunogenicity with the help of vaccine adjuvants is also being a consideration. In this light, AS03, MF59, and CpG 1018 are several approved adjuvants produced specifically for the COVID-19 vaccine [12].
The design of the vaccine includes the selection of antigens, vaccine platforms, and routes and regimens for vaccination [14]. The relative immunogenicity of vaccine-derived viral antigens, whether an immune adjuvant is needed, and the essence of protective immunity are determined by the choice of the vaccine platform. The suitability of a vaccine for a specific vaccination route and whether a prime-boost vaccination regimen is necessary to improve mediated protective immunity and stability of vaccine is also determined by these characteristics [14].
This section will outline the important facets of COVID-19 vaccine development that should be considered.
Antigens selection
Based on SARS/MERS experiences, the research vaccine development proposes S1/S2 protein subunits, receptor-binding domain (RBD), and S protein/gene as the most preferred target sites. To date, several potent neutralizing monoclonal antibodies (nAbs) to SARS-CoV-2 that target the RBD have entered clinical trials. Non-neutralizing antibodies are generated to S, E, and M proteins. However, unlike the S protein, M and E proteins have never been explored as vaccine targets alone against COVID-19 due to their poorly immunogenic for humoral responses [15]. Because of the uncertainly role of these non-neutralizing antibodies, as well as inadequately neutralizing antibodies in antibody-dependent enhancement (ADE) disease, the addition of other structural (N) and/or non-structural proteins as vaccine antigens may contribute to a more balanced response relating humoral and T cell-mediated immunity [15]. The inclusion of highly expressed proteins (e.g., N protein) or highly conserved functional proteins that have a key role in the life cycle of virus (e.g., RNA-dependent RNA polymerase) in a vaccine design can ensure that all emerging variant strains are targeted.
The routes and regimens of vaccination
The vaccination route is an integral part of vaccine strategies, especially important for mucosal pathogens such as SARS-CoV-2 and those pathogens against which optimal protection is provided not only to neutralizing antibodies but also to innate and adaptive cellular immunity.
The best opportunity to control and clear SARS-CoV-2 is the asymptomatic course of COVID-19 (2 to 12 days), which probably requires the presence of all the protective elements in the respiratory mucosa before the virus enters [14].
Emerging studies show that all or the majority of patients with COVID-19 have a strong and broad T-cell response in both CD4 and CD8, and some people develop a memory phenotype that heralds potential long-term immunity. In addition, milder patients of COVID-19 have more number tissue-resident memory T cells (TRM cells) in the respiratory tract than patients with severe patients [14, 16]. Evidence shows that the induction of such lung TRM cells depends on the vaccination route. The most common parenteral routes include subcutaneous (SC), intradermal (ID), and intramuscular (IM); while common mucosal routes are non-invasive oral and nasal. The respiratory mucosal route of vaccination is adept at strong inducing antibodies and lung TRM cell responses as well as macrophage-mediated trained immunity, whereas parenteral vaccination is unable to do so [14, 17].
Protein subunit, inactivated virus, and nucleic acid vaccines cannot be delivered through the respiratory mucosal route due to their need for potentially risky immune adjuvants and repeated delivery. By comparison, recombinant viral-vectored vaccines, particularly those using Ad5 and ChAd are safe and highly effective in the respiratory mucosal vaccination route [18].
Former studies in SARS-CoV patients have reported significant reductions in neutralizing antibody titers between 1 and 2 years after infection. Regularly, weak immunogenic vaccines require a repeated homologous vaccination regimen to be effective [19]. Since it is not yet known a COVID-19 vaccine strategy would be used or how long the protection caused by the vaccine will last in humans, a homologous or heterologous vaccination regimen may be needed to maintain the protection.
Vaccine platforms
Candidates for the COVID-19 vaccine come in a variety of compositions, from traditional whole-pathogen vaccines to new-generation vaccines. Classic vaccine platforms can consist of live attenuated virus, inactive virus, protein subunit, and virus-like particle. While next-generation platforms are including recombinant viral vector, nucleic acid-based, and antigen- presenting cells [11, 14, 20]. The different types of vaccine platforms have been shown in Fig. 1. Some platforms provide crucial advantages such as nucleic acid-based with antigen manipulation flexibility, and viral vectors offer their strong immune response, superior protein expression, and prolonged stability, whereas the recombinant protein-based development approach is easier to scale up using established manufacturing capabilities [12]. In Table 1 we list some of the most advanced COVID-19 vaccine candidates that have recently moved into clinical development. In the following, we aim to review different types of vaccines, their compositions, advantages, disadvantages, and potential limitations of vaccine development.
Live attenuated viral vaccines
Classically, live attenuated virus vaccines are produced by passing through cell culture until their pathogenicity is lost and only a mild infection is caused upon injection. Live attenuated vaccines can naturally provide two components required to alert and activate the host immune system: antigens and infection signals. Attenuated virus strains can be rationally designed by mutation or deletion of virulence genes. In this strategy, deletion of various non-structural proteins, as well as of the structural E protein, has been used to engineer vaccine strains of numerous coronaviruses [14, 21]. The E protein deletion leads to attenuation and production of an efficient vaccine strain, but the deformation of the attenuated phenotype has been recorded [22]. Consequently, the deletion of virulence factors can provide a preferred attenuation mechanism.
Codon deoptimization is another method for viral attenuation that noticeably slows the translation of the viral protein during infection. This method can produce a highly weak in vivo virus that can still replicate in vitro if the right viral protein is chosen for deoptimization [14]. For COVID-19, Calmette–Guérin (BCG), as a live attenuated vaccine, is under investigation in clinical trials (Table 1). BCG is an FDA-approved vaccine that has been developed against tuberculosis a century ago and is repurposed against SARS-CoV-2 infection now.
The main drawbacks associated with live attenuated vaccines are potential safety concerns. Live attenuated vaccines are usually more reactive than recombinant protein-based vaccines, and can infect or reverse the virulent strain in individuals with weakened immune systems.
Inactivated viral vaccines
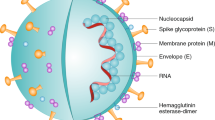
Physically or chemically inactivated viral vaccines are one of the oldest approaches to vaccine design that have been used successfully against various human viral infections such as polio, hepatitis A and influenza [23]. The principles of this method include injecting the inactivated virus into the host, which induces an immune response and then promotes a strong immunity against the virus. In a pandemic scenario, inactivated viruses can be produced and scaled up quickly using well-established infrastructure and techniques (Fig. 2) [14]. Contrasting their live attenuated counterparts, inactivated viral vaccines have few safety issues, and they convey a large range of native viral antigens, including surface antigens with conserved epitope conformations that can stimulate conformation-dependent responses to antibodies [24]. Because completely inactive viruses do not replicate, adjuvants and repeated administration are needed to stimulate the immune system and the full efficiency of these vaccines. Additionally, inactivated viral vaccines are poor inducers of cytotoxic CD8+ T cells that are likely to be needed for a successful COVID-19 vaccine, as are protein subunit vaccines. Such safety issues can be avoided by the use of TH1 cell-skewing modified alum or other adjuvants like CpG [25]. A phase III trial of BBIBP-CorV is currently underway by Sinopharm (ChiCTR2000034780).
Recombinant viral-vectored vaccines
Recombinant viral-vectored vaccines use a bioengineered viral vector that lake its reproduction ability to express and clone antigens derived from the target pathogen. Common vectors used in this platform are lentivirus, retroviruses, adenovirus, and adeno-associated virus (AAV). Given their genetic malleability, safety, and capacity to stimulate strong cellular immune responses without the need for an adjuvant, the non-replicating viral vaccines have been extensively studied and have a well-established record for infectious diseases. Also, a single dose of these viral vector-based vaccines can be sufficient for protection [26].
While the commercial method of producing viral vectors for gene therapy was created decades ago, the complexity of viruses needs optimization for particular products to satisfy quality and cost-effectiveness criteria. A bottleneck of virus vector production is the scaling-up of virus production capacity [10, 27]. To achieve scaling up, a suspension expression system cultured in a bioreactor may be used to replace the adherent cells grown in cell stacks [10]. The size of the filter pores is important, if the size of the pores of the chosen filter is small, virus particles will stick to the cell membrane, cell debris, and/or impurities of the host cell, and if the selected filter is large pore size, it will lead to insufficient removal of process-related impurities. Successful impurity removal, high recovery efficiency maintenance, and cost reduction for each dose depend on the optimization of the downstream process such as centrifugation, DNA digestion, size exclusion chromatography, ion-exchange chromatography, and tangential flow filtration methods [28]. Removal of residual host cell DNA and host cell proteins is another dilemma of the suspension virus construction process because DNA and proteins may adhere tightly to viral particles and be destroyed by chromatographic processes [28]. In the development of the production process, the biophysical characteristics of the individual virus type and the relationship between each stage must also be considered.
Early clinical trials data showed significant antibody and cell-mediated responses after a single dose of adenoviral vectored vaccines, Ad5‐nCoV and Ad26.CoV2. S. Since Ad26 is generally less immune than Ad5, repeated homologous or heterologous vaccinations are needed for successful immunity, as shown in human studies of the Ad26-HIV and Ad26-Ebola vaccines. However, the single parenteral performance of the vaccine Ad26-vectored COVID-19 provided robust safety in the non-human primate model SARS-CoV-2. Phase I (NCT04568811) and phase II (NCT04566770) trials are ongoing to evaluate the safety and immunogenicity of a booster dose six months after prime and the two-dose regimen. Moreover, a single-center, randomized and double-blinded trial is also undergoing testing the immunobridging between different manufacture scales and lot-to-lot consistency of the Ad5‐nCoV in different age groups (NCT04916886).
A phase 3 clinical trial has been done by Oxford University in cooperation with pharmaceutical company AstraZeneca to define the safety, efficacy, and immunogenicity of the non-replicating ChAdOx1 nCoV-19 (AZD1222) vaccine (NCT04536051) [14]. in February 2021, phase 4 clinical trials have been started for the non-replicating ChAdOx1 nCoV-19 vaccine.
AZD1222 has been licensed by the UK Medicines and Healthcare products Regulatory Agency (MHRA) to provide emergency immunization for adults aged 18 and over, and it is recommended to inject two doses at an interval of four to 12 weeks.
Protein subunit vaccines
The protein subunit vaccine uses full-length SARS-CoV-2 S protein or a protein fragment such as the RBD or combination of RBD with a carrier protein as the antigen for inducing a strong immune response against the virus (Fig. 2). The biggest downside of the recombinant protein vaccine is that due to the poor immunogenicity of proteins and peptides, this vaccine usually only triggers humoral immune responses and sometimes has only partial protection against viral infections. Therefore, these vaccines often require not only an adjuvant in the formulation but also repeated administration to establish immune memory. Besides, protein vaccines are not appropriate for respiratory mucosal vaccination. Similar to inactivated viral vaccines, unmodified alum as an adjuvant inhibits the immune response that is undesirable for host defense and may play a role in ADE of disease [29]. In this regard, a placebo-controlled 1/2 phase trial (NCT04368988) was assessing the immunogenicity of a full-length recombinant SARS-CoV-2 spike nanoparticle vaccine (NVX-CoV2373) with and without Matrix-M as the adjuvant [10]. The Novavax vaccine is currently in phase 3 trial.
Virus-like particles
Virus-like particles (VLPs), which form particles spontaneously assembled from several viral structural proteins, are gradually emerged as vaccine delivery agents. These multimeric structures can directly stimulate the immune system via imitating the 3D structure of native viruses. VLPs are good options for vaccine production due to the lack of infectious genetic material as well as functional proteins for virus entry. Compared to attenuated viruses, Virus-like particles are safer and more effective and have higher immunogenicity. Moreover, VLPs have outstanding adjuvant characteristics, and are able to induce both humoral and cellular immune responses. Considering these features, several commercial vaccines have been developed based on the VLPs platform [14]. In the case of coronaviruses, VLPs are formed when the viral proteins (S, E, and M with or without N) are co-expressed in eukaryotic producing cells. This results in active budding from the producer cells of VLPs that are structurally similar to the infectious virus but are non-infectious due to the lack of a viral genome. VLPs are capable to bind and enter into cells presenting ACE2 by the S protein on their surface in the same way as the parent virus [30]. Contrasting subunit vaccines, the S protein array on the VLPs surface crosslinks to the receptor of B cells and activates these cells directly, but VLPs often usually require an adjuvant and multiple doses, like inactivated and subunit viral vaccines. Nevertheless, the technology of VLPs is well established, the biology and safety of coronavirus VLPs are well known, and their large-scale construction is relatively simple [31].
RNA vaccines
Compared to other vaccine approaches, the mRNA-based COVID-19 vaccines are more attractive due to their low cost and rapid manufacturing process. The use of nanotechnology boosts the delivery of mRNA vaccines via the ID or IM route. In this light, antigen-encoding mRNA that coated with lipid nanoparticles (LNPs), as a stable lipid bilayer, can be effectively transferred in vivo into the host cells, which is an advantage over recombinant protein subunit vaccine platform [8]. After uptake into cells, mRNA prompts the expression of viral antigen in target cells, which in turn induces the adaptive immune system to produce neutralizing antibodies against the target antigen (Fig. 2). The mRNA vaccines are non-infectious and are synthesized without microbial molecules by in vitro transcription. These beneficial properties distinguish mRNA vaccines in terms of protection, effectiveness, and anti-vector immunity issues from live attenuated viral vaccines, inactivated viral vaccines, recombinant viral-vectored vaccines, and protein subunit vaccines. Therefore, these features enable rapid and inexpensive manufacturing and repeat vaccination. Another advantage of the RNA vaccine platform is likely induction of protective immunity by using a lower dose because, in self-replicating RNA vaccines, more vaccine antigen is expressed per cell [26]. However, there are concerns regarding the instability of delivering naked RNA, along with the size of the delivered molecules [32, 33].
RNA vaccines may also be associated with adverse effects, considering their strong immune capacity. RNA vaccines can lead to autoimmune disorders through the induction of the development of type I IFNs. In addition, because RNA vaccines contain a non-human polynucleotide, an unwanted immune response may be triggered, leading to extreme immune reactions and adverse effects [20].
A number of major biotechnology companies, such as BioNTech, Pfizer, Moderna, and CureVac, have developed COVID-19 vaccine using the advanced mRNA vaccine platform. mRNA-1273, which is produced by Moderna, encodes a perfusion-stabilized spike protein encapsulated in LNPs. Moderna mRNA-1273 was the first vaccine to enter phase I clinical trials (NCT04283461) and has shown promising results in preclinical studies in the fight against COVID-19 [34]. It has shown 94% efficiency in phase 3 clinical trials (NCT04470427) [35]. A phase 4 clinical trial (NCT04760132) has been launched to evaluate the effectiveness and safety of this SARS-CoV-2 vaccine [36]. Furthermore, a clinical trial (NCT04380701) has been designed to investigate the safety, efficacy, and immunogenicity of Pfizer’s nucleoside-modified mRNA candidates (BNT162a1, BNT162b1, BNT162b2, and BNT162c2) in preventing COVID-19 [37]. Phase 1/2 studies have revealed that, compared to Moderna's candidate vaccines, BNT162b1 induces a stronger CD8-T cell response, which can promote the production of CD4-T cells and neutralizing antibodies [38].
The phase 3 clinical trial (NCT04368728) shown that the lipid nanoparticle–formulated BNT162b2 vaccine in persons 12-to-15-year-old produced a greater immune response than in young adults, and had a favorable safety and efficacy against Covid-19 [39]. A two-dose regimen of this vaccine conferred 95% protection against SARS-CoV-2 in 16 years of age or older recipients [40].
DNA vaccines
For decades, recombinant plasmid DNA has been explored as a vaccine platform. DNA vaccines include a genetically engineered plasmid encoding the pathogen‐specific antigen, and a vector responsible for carrying the plasmid into the host cell. The injection needs to be accompanied by electroporation for efficient absorption of the plasmid into cells. After transduction, an engineered plasmid induces the expression of the vaccine antigen (Fig. 2). Plasmid DNA vaccines share many features with mRNA vaccines, such as safety, ease of manufacture, and scalability [41]. However, these vaccines are poorly immunogenic, requiring repeated administration and the addition of an adjuvant. DNA vaccines have been investigated in a broad variety of infectious diseases and have also been considered in the COVID‐19 pandemic.
A phase I clinical trial (NCT04336410) is designed by Inovio Pharmaceuticals to study the effect of a synthetic DNA vaccine expressing SARS-CoV-2 S protein (INO-4800) against COVID-19 [20]. Sun et al. used CRISPR/Cas9 system to generate mouse models expressing hACE2 to study the transmission and pathogenesis of SARS-CoV-2 and to develop a useful tool for evaluating COVID-19 vaccines [42].
DNA vaccines are often concerned with certain drawbacks and side effects because they are genetically modified. In DNA vaccines, only protein antigens can be utilized as immunogens. The risk of the formation of anti-DNA antibodies and the creation of dysplasia by prompting mutations in the host genome are also two examples of the adverse effects of plasmid DNA vaccines [20].
Antigen-presenting cells
Antigen-presenting cells are the important elements in the response of the immune system to a vaccine. Traditionally, dendritic cells are usually taken from the person, then enlarged and manipulated to deliver the desired antigen, and injected back into the same person. This is expensive and too time-consuming for a large-scale vaccine. This has led to the development of artificial antigen-presenting cells (aAPCs) that by introducing the pathogen‐associated antigen to the adaptive immune cells contribute to the antiviral immune response. aAPCs bypass the need to harvest patient dendritic cells. These vaccines are lentivirally modified APCs that express the proteins of the virus and subsequently activate T cells and induce an immune response against the virus [43]. In preclinical and clinical studies, aAPCs vaccines have been considered for the development of the COVID-19 vaccine [44]. A Phase I clinical trial (NCT04299724), is using inactivated aAPCs expressing conserved structural and protease epitopes of SARS-CoV-2 to evaluate the safety of this vaccine in healthy and COVID-19 positive volunteers [45]. The deployment of these vaccines on a large scale is prevented by extra cold chain requirements for cell-based vaccines and injection methods, particularly because repeated administration is required for a desirable response. Vaccine platforms and targeted selected antigens for SARS-CoV-2, and their advantages and disadvantages are summarized in Table 2.
Nanotechnology and COVID-19
Nanotechnology is an area that is embracing rapidly in many sectors, ranging from healthcare and medicine to electronics and construction. In medicine, it is expected to revolutionize diagnostics, drug delivery, gene therapy, regenerative medicine, and the treatment of various diseases. It can also be considered to develop drugs with successful therapeutic effects and lower side effects. [46]. Nanoparticles (NPs) such as metal, peptide, graphene oxide, small interfering RNA (siRNA), carbon quantum dots (CQDs), and organic materials have been suggested as antiviral agents [47,48,49]. Today, nanotechnology can establish a therapeutic approach for the treatment of COVID-19 [50]. NPs can deliver drugs to the target organ accurately at the proper time [51]. NPs that contain immune-regulating molecules and antioxidants (adenosine and α-tocopherol, respectively) can deliver their therapeutic contents to inflammation sites and reduce inflammation, cytokine reactions, and oxidative stress associated with COVID-19 [52, 53].
NPs have also developed in nanomedicine as identification factors and direct inhibitors of various microbes and cancer cells. Moreover, NPs play an essential role in vaccines design, diagnosis, follow-up, and therapeutic responses using non-invasive imaging techniques. Considering the size of SARS-CoV-2 that is in the nanoscale, nanotechnology can be used to fight against COVID-19 [54]. Drug NPs can provide new and cost-effective solutions for COVID-19 treatment by increasing biocompatibility, biodegradation, and being environmentally friendly [55]. In a computational study, ACE2-based peptide inhibitors were proposed for SARS-CoV-2 blocking. In this study, nanoparticles have been considered as carriers to which multiple peptides bind to their surface. The authors hypothesized that these designed peptide domain-based NPs mimic the binding domain of the virus to the ACE2 receptor and could be used as inhaled therapeutics, preventing the virus activation in the lungs [56].
One of the main objectives in COVID-19 vaccine development is the more effective stimulus of T and B immune cells against the virus and the production of the next-generation vaccine with greater immunogenicity [57]. Nevertheless, poor immunogenicity is created because of the rapid degradation of antigens in the body and failing of DNA- and RNA-based vaccines to reach the target sites [58]. Therefore, designing the COVID-19 vaccine with more protection, durability, immunization, and more accessibility to target cells is important. In this regard, nanoparticle vaccine carriers can be considered [59].
Nano-based vaccine strategists for coronaviruses
Viromimetic nanoparticle vaccine against CoVes
Virus-like nanoparticles (VLNPs) have been proven to be effective in stimulating the immune system, so much attention has been paid to the development of VLNPs to combat corona disease [60]. These types of NPs can improve vaccine performance, immunization, and targeted delivery. VLNPs play a role with greater vaccine efficacy, rapid proteolytic inhibition of antigens, increased antigen uptake, antigen release control, and non-toxicity [61]. These NPs are composed of capsids, which are structural and non-infectious proteins of viruses, and have been modified for use in nanotechnology. VLNPs can be produced from productive infections of host material or by recombinant protein expression and self-assembly [62]. One of the main properties of VLNPs is that they can contain additional proteins synthesized from multiple antigens [63, 64] and have a protective function against viruses and heterologous antigens [65]. VLNPs can accommodate the additional proteins expression or the endogenous expression of multiple antigens as carriers on their surface [66]. Also, these NPs with high surface energy cause severe adhesion of biological molecules. These actions are similar to the effect of the virus on stimulating the immune system to produce antibodies for eliminating the infection [67, 68]. Thus, VLNPs have special value in vaccine production. Peptide-based NPs are also being studied in the vaccine to fight against the SARS-CoV-2 [69]. Antibody production and inhibition of coronavirus function have been observed in self-assembled peptide NPs using the SARS-CoV-1 S protein [69].
In a study on VLNPs in mice, the MERS-CoV S protein NPs vaccine with the adjuvant combination of the matrix protein (M1) shown efficacy in inhibiting the MERS-CoV replication in the lungs of mice. The high titer of neutralizing antibody against S protein indicates a protective state against the virus in mice [70]. Thus, designed VLNPs with the S protein can be effective not only against the MERS-CoV but also against the SARS-CoV-2 because both viruses use the same mechanism of entry into host cells and cause infection [71].
Gold nanoparticles vaccine against CoVes
Gold NPs (AuNPs) are more effective in radiation therapy, cancer cell killing, cell and protein labeling, and drug delivery to target cells [72]. Therefore, conjugated AuNPs with a type of coronavirus called transmissible gastroenteritis virus (TGEV) has been used to assess immunity in mice and rabbits [73]. The results showed that immunization against TGEV was performed by significantly increasing the antibody titer, increasing the concentration of IFN-γ, tenfold expansion of T cells, and macrophage respiratory activity. The researchers also developed the anti-MERS-CoV vaccine by combining ferritin-based NP assembly mediated by RNA. They concluded that CD4+ T cell expression is induced and eventually led to IFN-γ and TNF-α expression. Therefore, gold-based NPs can be a good option for preparing a vaccine against coronavirus [74]. In one study in SARS-CoV-infected mice, AuNPs and Toll-like receptor (TLR) agonists with recombinant S protein were evaluated [75]. The AuNPs-adjuvanted S protein vaccine has been claimed to be very effective in inducing an IgG immune response, however, the AuNPs-adjuvanted TLR vaccine does not affect the induction and production of protective antibodies and eosinophilic infiltration [75].
Polymer-based materials vaccine against CoVes
Polymeric molecules are composed of repetitive and long units and have found many applications in industry and medicine due to their special properties [50]. Polymers are used as delivery systems and adjuvants to improve the performance of vaccines for increasing immune response, safety, and effectiveness. Also, the antigen is encapsulated in polymer structures and creates a barrier to rapid degradation in vivo [76]. Polymer-Based (PB)-NPs are synthesized with different formulations and have linear, three-dimensional, and branched network structures. In this group of NPs, size, shape, and surface charge play a key role in controlling the release of the loaded compound [77, 78]. PB-NPs have a special place in the preparation of VLNP anti-MERS-CoV, which it could mimic the function of the virus [79]. Therefore, using PB-NPs to design effective vaccines against COVID-19 could probably be considered.
Lipid nanoparticles vaccine against CoVes
Lipid-based nanoparticles have different types and physicochemical properties depending on their formulations [80]. These NPs include liposomes, niosomes, transfersomes, solid lipid nanoparticles (SLN), and nanostructured lipid carriers (NLC). In the lipid-based formulations for each of these NPs, there are different lipid compounds such as phospholipids, cholesterol, essential oils, surfactants, solid fats, and edge activators [81]. LNPs are non-viral carriers used to deliver nucleic acid vaccines to target tissues [82]. These NPs are used in gene therapy, protein replacement therapy, and mRNA-based vaccines in cancer and infectious diseases treatment [83]. Nanostructured lipid carriers (NLCs) are a group of LNPs that are used as vectors of mRNA-based vaccines and have a little toxic effect on the vaccine recipient [84]. Today, the best protection against COVID-19 is the production of RNA-based vaccines [34]. Therefore, several preclinical studies have evaluated the efficacy and immunogenicity of LNP-mRNA vaccines that encode the SARS-CoV-2 S protein or S receptor-binding domain [85,86,87,88,89]. The results of a study on the virus S protein RNA encapsulated in the LNP vaccine in mice showed an enhanced immune response against SARS-CoV-2 [87]. As we mentioned before, the LNP-mRNA vaccines were developed by companies like Pfizer and Moderna. The nanoparticle-based vaccines produced by institutions and pharmaceutical manufacturers list in Table 1.
Advantages and disadvantages of applying nanoparticle-based vaccines
One of the advantages of using NPs in vaccine preparation is an enhancement of the antigenicity of conjugated or adsorbed agents [90]. In this case, they can act like viruses in the form of a pathogen [91]. NPs can be also effective in stimulating innate and adaptive immune systems. Finally, NPs play an essential role in antigen delivery as antigen carriers due to their efficient cell targeting and controlled release properties [92]. However, NPs-based vaccines have some limitations. One of the major limitations of the use of NPs in the design of the vaccines is their cellular toxicity and the need for an adjuvant to make the vaccine more effective [93]. Also, the size, shape, charge, and surface area of NPs have a significant effect on vaccine performance [93, 94]. Other disadvantages of the coronavirus NPs-based vaccines include the need for multiple doses, delayed immune response, and side effects [95].
Potential side effects of COVID-19 vaccine
No vaccine is completely free from the risks of adverse events or complications [96]. The rapid development process and lack of sufficient follow-up time after vaccination have raised public concern related to the safety of current COVID-19 vaccine candidates. At present, new regulatory frameworks were established that authorized the expedited data review and acceptance of new COVID-19 vaccines without safety data. Many of the new vaccines are completely based on new technologies that have never been used for a licensed vaccine before such as RNA and DNA-based vaccines [97]. Also, no vaccine against coronaviruses had ever been licensed for use in humans before [97]. Therefore, there is some level of hesitation related to side effects and potential adverse reactions of new COVID-19 vaccines in the global community.
Increasing studies have been designed to evaluate the safety risks of SARS-CoV-2 vaccines based on articles and results posted on various websites and databases. A meta-analysis study investigated 87 publications with safety data from clinical trials and post-authorization researches of 19 SARS-CoV-2 vaccines on 6 different platforms [98]. Obtained results from this analysis showed that inactivated vaccines, protein subunit vaccines, and DNA vaccines have significantly lower rates of local and systemic reactions compared with RNA vaccines, non-replicating vector vaccines, and granular vaccines. Pain at the injection site was the most frequent solicited local reaction, and fatigue and headache were the most frequent systemic reactions. The frequency of reported serious vaccine-related side effects was lower than 0.1% and balanced between recipients [98]. In a meta-analysis study from 25 randomized clinical trials, the clinical characteristics of COVID-19 vaccines were investigated to better assess their efficacy and adverse effects [99]. The study included 58,889 cases and 46,638 controls who received the COVID-19 vaccine or a placebo, respectively. The highest efficacy was related to adenovirus-vectored and mRNA-based COVID19 vaccines after the first and second doses. The mRNA-based vaccines indicated higher adverse effects in reactogenicity, including site pain, swelling, redness, fever, headache, fatigue, induration, vomiting, myalgia, chills, and pruritus. Severe harmful side effects such as anaphylactic shock and allergic reactions were not significant for these vaccines. Compared to other vaccines, adenovirus vector vaccines are associated with increased diarrhea and arthralgia. As well, one case of anaphylactic shock was reported for this vaccine [99]. Various cases of pulmonary embolism and deep vein thrombosis were reported as rare events of the Oxford-AstraZeneca vaccine, leading to the temporary discontinuation of the vaccine’s use in several countries and the introduction of age-specific rollout in other countries/regions vaccination. Although, so far, the data is too limited and anecdotal to provide clear causal evidence [100]. In like manner, Johnson & Johnson's vaccine was temporarily suspended by the FDA in April 2021, because many people showed rare blood-related problems due to thrombosis and thrombocytopenia syndrome, causing cerebral venous sinus thrombosis [99].
Overall, an important aspect of COVID-19 vaccine development is ensuring that potential safety risks and developing complications are identified and weighed against the potential benefits. In this regard, more and more research and long-term population-level monitoring are strongly recommended, to further strengthen the safety profile of the various COVID-19 vaccines.
Conclusion and future perspective
Nowadays, SARS-CoV-2 has caused an international health emergency. From the earliest days of the recent pandemic, researchers have focused on finding a way to treat, prevent, and overcome COVID-19 using a variety of computational and experimental techniques. In this situation, along with trying to find effective treatments for COVID-19, vaccination is the best way to control the disease. In the light of coronaVac development, different approaches from nucleic acids and protein subunits to virus-like nanoparticles have been used. None of the designed COVID-19 vaccines provide 100% protection. This is why, even with vaccination, there is a chance of infection, but it is probably asymptomatic or very mild, and the chances of serious illness and death are actually very low. In cases of pandemics such as COVID-19, in addition to the common criteria for successful vaccine development, including safety, efficacy, and duration of safety, rapid manufacturing of a vaccine with a very high production capacity, distributing, and administering the vaccine to the vulnerable population are other important challenges. Another bottleneck in the process of vaccine development against COVID-19 is constant mutational changes of the SARS-CoV-2 structure. At the time of writing, Lambda variant SARS-CoV-2 has emerged with increased infectivity and immune escape from neutralizing antibodies elucidate by COVID-19 vaccines. Hence, despite the increasing speed of immunization against COVID-19 worldwide, the immune escape variant has raised concerns about the efficacy of the vaccine. Therefore, universities and companies involved with the development of new vaccines should be accompanied by strict genomic and proteomic monitoring to rapidly identify mutations and analyze their effects on the transmission rate, severity of pathogenicity, and immune escape for better COVID-19 vaccine development and pandemic management.
Data availability
Not applicable.
Abbreviations
- ACE2:
-
Angiotensin converting enzyme 2
- APC:
-
Antigen-presenting cell
- ChAdOx1:
-
Chimpanzee adenoviral vector 1
- COVID-19:
-
Coronavirus disease-19
- CQD:
-
Carbon quantum dots
- LNP:
-
Lipid nanoparticles
- ID:
-
Intradermal
- IM:
-
Intramuscular
- MERS:
-
Middle east respiratory syndrome
- RBD:
-
Receptor binding domain
- SARS-CoV-2:
-
Severe acute respiratory syndrome coronavirus-2
- SC:
-
Subcutaneous
- siRNA:
-
Small interfering RNA
- TLR:
-
Toll-like receptor
- VLP:
-
Virus-like particle
References
Wilder-Smith A, Freedman DO (2020) Isolation, quarantine, social distancing and community containment: pivotal role for old-style public health measures in the novel coronavirus (2019-nCoV) outbreak. J Travel Med 27(2):1–4
Jafari A et al (2020) Cancer care management during the COVID-19 pandemic. Risk Manag Healthc Policy 13:1711–1721
https://covid19.who.int/. Accessd 20 Dec 2021
Jean S-S, Lee P-I, Hsueh P-R (2020) Treatment options for COVID-19: the reality and challenges. Microbiol Immunol Infect 53:436–443
Control CfD Prevention (2006) Vaccine preventable deaths and the global immunization vision and strategy, 2006–2015. MMWR Morb Mortal Wkly Rep 55(18):511–515
Chakraborty S et al (2021) SARS-CoV-2 vaccines in advanced clinical trials: where do we stand. Adv Drug Deliv Rev 172:314–338
https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines. Accessed 20 Dec 2021
Salian VS et al (2021) COVID-19 transmission, current treatment, and future therapeutic strategies. Mol Pharm 18:754–771
Prompetchara E, Ketloy C, Palaga T (2020) Immune responses in COVID-19 and potential vaccines: lessons learned from SARS and MERS epidemic. Asian Pac J Allergy Immunol 38:1–9
Wang J, Peng Y, Xu H, Cui Z, Williams RO (2020) The COVID-19 vaccine race: challenges and opportunities in vaccine formulation. AAPS PharmSciTech 21:1–12
Calina D et al (2020) Towards effective COVID-19 vaccines: updates, perspectives and challenges. Int J Mol Med 46:3–16
Chauhan G et al (2020) Nanotechnology for COVID-19: therapeutics and vaccine research. ACS Nano 14:7760–7782
Zhou Y et al (2020) Network-based drug repurposing for novel coronavirus 2019-nCoV/SARS-CoV-2. Cell Dis 6:1–18
Jeyanathan M et al (2020) Immunological considerations for COVID-19 vaccine strategies. Nat Rev Immunol 20:615–632
Dai L, Gao GF (2021) Viral targets for vaccines against COVID-19. Nat Rev Immunol 21:73–82
Grifoni AWD et al (2020) Targets of T cell responses to SARS-CoV-2 coronavirus in humans with COVID-19 disease and unexposed individuals. Cell 181:1489–1501
Moreno-Fierros LG-SI, Rosales-Mendoza S (2020) Development of SARS-CoV-2 vaccines: should we focus on mucosal immunity? Expert Opin Biol Ther 20:831–836
Afkhami SYY, Xing Z (2016) Methods and clinical development of adenovirus-vectored vaccines against mucosal pathogens. Mol Ther Methods Clin Dev 3:16030–16039
Cao WCLW, Zhang PH, Zhang F, Richardus JH (2007) Disappearance of antibodies to SARS-associated coronavirus after recovery. New Engl J Med 357:1162–1163
Esmaeilzadeh A, Elahi R (2020) Immunobiology and immunotherapy of COVID-19: a clinically updated overview. J Cell Physiol 236(4):2519–2543
Netland JDM et al (2010) Immunization with an attenuated severe acute respiratory syndrome coronavirus deleted in E protein protects against lethal respiratory disease. Virology 399:120–128
Jimenez-Guardeño JMR-NJ et al (2015) Identification of the mechanisms causing reversion to virulence in an attenuated SARS-CoV for the design of a genetically stable vaccine. PLoS Pathog 11:e1005215
Vellozzi CBD et al (2009) Safety of trivalent inactivated influenza vaccines in adults: background for pandemic influenza vaccine safety monitoring. Vaccine 27(15):2114–2120
Ul Qamar MT et al (2019) Epitope-based peptide vaccine design and target site depiction against Middle East Respiratory Syndrome Coronavirus: an immune-informatics study. J Transl Med 17:362–376
Del Giudice GRR, Didierlaurent AM (2018) Correlates of adjuvanticity: a review on adjuvants in licensed vaccines. Semin Immunol 39:14–21
van Riel D, dWE, (2020) Next-generation vaccine platforms for COVID-19. Nat Mater 19:810–812
Farahani M et al (2022) Molecular pathways involved in COVID-19 and potential pathway-based therapeutic targets. Biomed Pharmacother 145:1–24
Gramer MJ (2014) Product quality considerations for mammalian cell culture process development and manufacturing. Adv Biochem Eng Biotechnol 139:123–166
Diamond MSPT (2020) The challenges of vaccine development against a new virus during a pandemic. Cell Host Microbe 27:699–703
DA Naskalska A et al (2018) Novel coronavirus-like particles targeting cells lining the respiratory tract. PLoS ONE 13:e0203489
Rauch SJE, Schmidt KE, Petsch B (2018) New vaccine technologies to combat outbreak situations. Front Immunol 9:1963
Wadhwa A et al (2020) Opportunities and challenges in the delivery of mRNA-based vaccines. Pharmaceutics 12(2):102–128
Saif LJ (2020) Vaccines for COVID-19: perspectives, prospects, and challenges based on candidate SARS, MERS, and animal coronavirus vaccines. Euro Med J 10:200324
Frederiksen LSF, Zhang Y, Foged C, Thakur A (2020) The long road toward COVID-19 herd immunity: vaccine platform technologies and mass immunization strategies. Front Immunol 11:1817–1842
COVID MM. vaccine candidate meets its primary efficacy endpoint in the first interim analysis of the phase 3 COVE study. Moderna. accessed 29 Jan 2021
https://clinicaltrials.gov/ct2/show/NCT04283461?term=NCT04283461&draw=2&rank=1. Accessed 1 Dec 2020
https://clinicaltrials.gov/ct2/show/NCT04380701?term=NCT04380701&draw=2&rank=1. Accessed 25 Nov 2020
Sahin U et al (2020) COVID-19 vaccine BNT162b1 elicits human antibody and TH 1 T cell responses. Nature 586:594–599
Frenck RW Jr et al (2021) Safety, immunogenicity, and efficacy of the BNT162b2 Covid-19 vaccine in adolescents. N Engl J Med 385:239–250
Polack FP et al (2020) Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med 383:2603–2615
Hobernik DBM (2018) DNA vaccines—how far from clinical use? Int J Mol Sci 19(11):3605–3632
Sun S-H et al (2020) A mouse model of SARS-CoV-2 infection and pathogenesis. Cell Host Microbe 28(1):124–133
Li HSS et al (2017) Artificial human antigen-presenting cells are superior to dendritic cells at inducing cytotoxic T-cell responses. Immunol 152:462–471
Huang LRY et al (2020) SARS-CoV-2 vaccine research and development: conventional vaccines and biomimetic nanotechnology strategies. Asian J Pharm Sci 16(2):136–146
Al-Kassmy J, Pedersen J, Kobinger G (2020) Vaccine candidates against coronavirus infections. Where does COVID-19 stand? Viruses 12:861–878
Parhizkar M et al (2018) Latest developments in innovative manufacturing to combine nanotechnology with healthcare. Nanomedicine 13(1):5–8
Hu C-MJ et al (2017) Nanoparticulate vacuolar ATPase blocker exhibits potent host-targeted antiviral activity against feline coronavirus. Sci Rep 7:1–11
Jiang Y et al (2016) Progress and perspective of inorganic nanoparticle-based siRNA delivery systems. Expert Opin Drug Deliv 13:547–559
Ye S et al (2015) Antiviral activity of graphene oxide: how sharp edged structure and charge matter. ACS Appl Mater Interfaces 7:21571–21579
Muhammad W, Zhai Z, Gao C (2020) Antiviral activity of nanomaterials against coronaviruses. Macromol Biosci 20(10):2000196
Kingsley JD et al (2006) Nanotechnology: a focus on nanoparticles as a drug delivery system. J Neuroimmune Pharmacol 1:340–350
Merad M, Martin JC (2020) Pathological inflammation in patients with COVID-19: a key role for monocytes and macrophages. Nat Rev Immunol 20:355–362
Dormont F et al (2020) Squalene-based multidrug nanoparticles for improved mitigation of uncontrolled inflammation in rodents. Sci Adv 6:eaaz5466
Sportelli MC et al (2020) Can nanotechnology and materials science help the fight against SARS-CoV-2? Nanomaterials 10:802–813
Chauhan DS et al (2020) Comprehensive review on current interventions, diagnostics, and nanotechnology perspectives against SARS-CoV-2. Bioconjug Chem 31:2021–2045
Han Y, Král P (2020) Computational design of ACE2-based peptide inhibitors of SARS-CoV-2. ACS Nano 14:5143–5147
Pandey SC et al (2020) Vaccination strategies to combat novel corona virus SARS-CoV-2. Life Sci 256:117956
Brisse M (2020) Emerging concepts and technologies in vaccine development. Front Immunol 11:2578–2599
Heinrich MA, Martina B, Prakash J (2020) Nanomedicine strategies to target coronavirus. Nano Today 35:100961–100981
Roldão A et al (2010) Virus-like particles in vaccine development. Expert Rev Vaccines 9:1149–1176
Pati R, Shevtsov M, Sonawane A (2018) Nanoparticle vaccines against infectious diseases. Front Immunol 9:2224–2239
Pokorski JK, Steinmetz NF (2011) The art of engineering viral nanoparticles. Mol Pharm 8:29–43
Strable E, Finn M (2009) Chemical modification of viruses and virus-like particles. Curr Top Microbiol Immunol 327:1–21
Patel KG, Swartz JR (2011) Surface functionalization of virus-like particles by direct conjugation using azide−alkyne click chemistry. Bioconjug Chem 22:376–387
Grgacic EV, Anderson DA (2006) Virus-like particles: passport to immune recognition. Methods 40:60–65
Moon JJ et al (2012) Enhancing humoral responses to a malaria antigen with nanoparticle vaccines that expand Tfh cells and promote germinal center induction. PNAS 109:1080–1085
Tenzer S et al (2013) Rapid formation of plasma protein corona critically affects nanoparticle pathophysiology. Nat Nanotechnol 8:772–781
Schöttler S et al (2016) Protein adsorption is required for stealth effect of poly (ethylene glycol)-and poly (phosphoester)-coated nanocarriers. Nat Nanotechnol 11:372–377
Pimentel TA et al (2009) Peptide nanoparticles as novel immunogens: design and analysis of a prototypic severe acute respiratory syndrome vaccine. Chem Biol Drug Des 73:53–61
Coleman CM et al (2017) MERS-CoV spike nanoparticles protect mice from MERS-CoV infection. Vaccine 35:1586–1589
Gurunathan S et al (2020) Antiviral potential of nanoparticles—can nanoparticles fight against coronaviruses? Nanomaterials 10(9):1645–1673
Khan A, Rashid R, Murtaza G, Zahra A (2014) Gold nanoparticles: synthesis and applications in drug delivery. Trop J Pharm Res 13:1169–1177
Staroverov S et al (2011) Immunostimulatory effect of gold nanoparticles conjugated with transmissible gastroenteritis virus. Bull Exp Biol Med 151(4):436–439
Kim Y-S et al (2018) Chaperna-mediated assembly of ferritin-based Middle East respiratory syndrome-coronavirus nanoparticles. Front Immunol 9:1093
Sekimukai H et al (2020) Gold nanoparticle-adjuvanted S protein induces a strong antigen-specific IgG response against severe acute respiratory syndrome-related coronavirus infection, but fails to induce protective antibodies and limit eosinophilic infiltration in lungs. Microbiol Immunol 64:33–51
Han J et al (2018) Polymer-based nanomaterials and applications for vaccines and drugs. Polymers 10:31–44
Piluso S, Halifa Soultan A, Patterson J (2017) Molecularly engineered polymer-based systems in drug delivery and regenerative medicine. Curr Pharm Des 23:281–294
Kamaly N et al (2012) Targeted polymeric therapeutic nanoparticles: design, development and clinical translation. Chem Soc Rev 41:2971–3010
Lin LCW et al (2019) Viromimetic STING agonist-loaded hollow polymeric nanoparticles for safe and effective vaccination against Middle East respiratory syndrome coronavirus. Adv Funct Mater 29:1807616
Puri A et al (2009) Lipid-based nanoparticles as pharmaceutical drug carriers: from concepts to clinic. Crit Rev Therap Drug Carrier Syst 26(6):523–580
Tapeinos C, Battaglini M, Ciofani G (2017) Advances in the design of solid lipid nanoparticles and nanostructured lipid carriers for targeting brain diseases. J Control Release 264:306–332
Whitehead KA, Langer R, Anderson DG (2009) Knocking down barriers: advances in siRNA delivery. Nat Rev Drug Dis 8:129–138
Samaridou E, Heyes J, Lutwyche P (2020) Lipid nanoparticles for nucleic acid delivery: current perspectives. Adv Drug Del Rev 154:37–63
Gómez-Aguado I et al (2020) Nanomedicines to deliver mRNA: state of the art and future perspectives. Nanomaterials 10:364–401
Laczkó D et al (2020) A single immunization with nucleoside-modified mRNA vaccines elicits strong cellular and humoral immune responses against SARS-CoV-2 in mice. Immunity 53:724–732
Lu J et al (2020) A COVID-19 mRNA vaccine encoding SARS-CoV-2 virus-like particles induces a strong antiviral-like immune response in mice. Cell Res 30:936–939
McKay PF (2020) Self-amplifying RNA SARS-CoV-2 lipid nanoparticle vaccine candidate induces high neutralizing antibody titers in mice. Nat commun 11:1–7
Tai W et al (2020) A novel receptor-binding domain (RBD)-based mRNA vaccine against SARS-CoV-2. Cell Res 30:932–935
Zhang N-N et al (2020) A thermostable mRNA vaccine against COVID-19. Cell 182:1271–1283
Reed SG, Orr MT, Fox CB (2013) Key roles of adjuvants in modern vaccines. Nat Med 19:1597–1608
Demento SL et al (2011) Pathogen-associated molecular patterns on biomaterials: a paradigm for engineering new vaccines. Trends Biotechnol 29:294–306
Demento SL et al (2012) Role of sustained antigen release from nanoparticle vaccines in shaping the T cell memory phenotype. Biomaterials 33:4957–4964
Kato T, Takami Y, Deo VK, Park EY (2019) Preparation of virus-like particle mimetic nanovesicles displaying the S protein of Middle East respiratory syndrome coronavirus using insect cells. J Biotechnol 306:177–184
Raghuwanshi D et al (2012) Dendritic cell targeted chitosan nanoparticles for nasal DNA immunization against SARS CoV nucleocapsid protein. Mol Pharm 9:946–956
Badgujar KC, Badgujar VC, Badgujar SB (2020) Vaccine development against coronavirus (2003 to present): an overview, recent advances, current scenario, opportunities and challenges. Diabetes Metab Syndr 14:1361–1376
Spencer JP, Pawlowski RHT, Thomas S (2017) Vaccine adverse events: separating myth from reality. Am Fam Phys 95:786–794
Krammer F (2020) SARS-CoV-2 vaccines in development. Nature 586:516–527
Wu Q, Dudley MZ, Chen X, Bai X, Dong K, Zhuang T, Salmon D, Yu H (2021) Evaluation of the safety profile of COVID-19 vaccines: a rapid review. BMC Med 19:1–16
Pormohammad A (2021) Efficacy and safety of COVID-19 vaccines: a systematic review and meta-analysis of randomized clinical trials. Vaccines 9:467–487
Wise J (2021) Covid-19: European countries suspend use of Oxford-AstraZeneca vaccine after reports of blood clots. BMJ 372:n699
Funding
This review received no external funding.
Author information
Authors and Affiliations
Contributions
Conceptualization, AJ and MRT.; validation, MRT, MAA, YR; investigation, AJ, FD, ZN; writing—original draft preparation, AJ, FD, ZN; writing—review and editing, AJ, MA; visualization, YR; supervision, MRT. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest regarding the publication of this article.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent to publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jafari, A., Danesh Pouya, F., Niknam, Z. et al. Current advances and challenges in COVID-19 vaccine development: from conventional vaccines to next-generation vaccine platforms. Mol Biol Rep 49, 4943–4957 (2022). https://doi.org/10.1007/s11033-022-07132-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-022-07132-7