Abstract
Background
Cervical cancers are usually treatable if detected in early stages by a combination of therapies. However, the prognosis of cervical cancer patients with metastasis remains unfavorable due to the fact that most of the cervical carcinomas are either resistant to anticancer drugs or show signs of relapse after initial treatment. Therefore, it is important to control the chemoresistance as it is the key to develop effective treatment options for cervical cancer.
Objective
The current study aimed at evaluating the differential responses of cervical cancer cells to anti-cancer drugs and assessed whether the differences in the expression profiles of antioxidant genes regulated by nuclear factor erythroid-2-related factor 2 (NRF2), led to the variations in the sensitivities of the cancer cells to treatment.
Methodology
Three cervical cancer cell lines were investigated for their differences in NRF2 pathway by measuring the gene expression and enzyme activity. The differences in the sensitivity to anti-cancer drugs and variation in ROS profile was also evaluated. The addition of exogenous drugs to manipulate the intracellular ROS and its effect on NRF2 pathway genes was also investigated.
Results
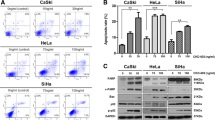
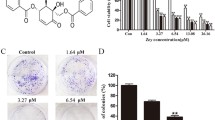
HeLa and SiHa cells were more sensitive to cisplatin and oxaliplatin treatment than C33A cells. HeLa and SiHa cells had significantly lower NRF2 gene levels, NQO1 enzyme activity and basal GSH levels than C33A cells. Levels of ROS induced were higher in HeLa than C33A cells.
Conclusion
Overall, the differences in the cellular levels of antioxidant regulatory genes led to the differential response of cervical cancer cells to anti-cancer drugs.






Similar content being viewed by others
Data availability
The data that support the findings of this study are available on request from the corresponding author.
References
Arbyn M, Weiderpass E, Bruni L, de Sanjosé S, Saraiya M, Ferlay J, Bray F (2020) Estimates of incidence and mortality of cervical cancer in 2018: a worldwide analysis. Lancet Glob Health 8(2):e191-203. https://doi.org/10.1016/S2214-109X(19)30482-6
de Martel C, Georges D, Bray F, Ferlay J, Clifford GM (2020) Global burden of cancer attributable to infections in 2018: a worldwide incidence analysis. Lancet Glob Health 8(2):e180–e190. https://doi.org/10.1016/S2214-109X(19)30488-7
Almeida AM, Queiroz JA, Sousa F, Sousa  (2019) Cervical cancer and HPV infection: ongoing therapeutic research to counteract the action of E6 and E7 oncoproteins. Drug Discovery Today 24(10):2044–2057. https://doi.org/10.1016/j.drudis.2019.07.011
Makovec T (2019) Cisplatin and beyond: molecular mechanisms of action and drug resistance development in cancer chemotherapy. Radiol Oncol 53(2):148. https://doi.org/10.2478/raon-2019-0018
Ebrahimi S, Soltani A, Hashemy SI (2019) Oxidative stress in cervical cancer pathogenesis and resistance to therapy. J Cell Biochem 120(5):6868–6877. https://doi.org/10.1002/jcb.28007
Hong JH, Tsai CS, Lai CH, Chang TC, Wang CC, Chou HH, Lee SP, Hsueh S (2004) Recurrent squamous cell carcinoma of cervix after definitive radiotherapy. Int J Radiat Oncol*Biol*Phys 60(1):249–257. https://doi.org/10.1016/j.ijrobp.2004.02.044
Hasan S, Taha R, El Omri H (2018) Current opinions on chemoresistance: an overview. Bioinformation 14(2):80. https://doi.org/10.6026/97320630014080
Fodale V, Pierobon M, Liotta L, Petricoin E (2011) Mechanism of cell adaptation: when and how do cancer cells develop chemoresistance? Cancer J (Sudbury Mass) 17(2):89. https://doi.org/10.1097/PPO.0b013e318212dd3d
Aoudjit F, Vuori K (2012) Integrin signaling in cancer cell survival and chemoresistance. Chemother Res Pract. https://doi.org/10.1155/2012/283181
Yang H, He L, Kruk P, Nicosia SV, Cheng JQ (2006) Aurora-A induces cell survival and chemoresistance by activation of Akt through a p53-dependent manner in ovarian cancer cells. Int J Cancer 119(10):2304–2312. https://doi.org/10.1002/ijc.22154
Mitsuishi Y, Motohashi H, Yamamoto M (2012) The Keap1–Nrf2 system in cancers: stress response and anabolic metabolism. Front Oncol 2:200. https://doi.org/10.3389/fonc.2012.00200
Kobayashi M, Yamamoto M (2005) Molecular mechanisms activating the Nrf2-Keap1 pathway of antioxidant gene regulation. Antioxid Redox Signal 7(3–4):385–394. https://doi.org/10.1089/ars.2005.7.385
Jaramillo MC, Zhang DD (2013) The emerging role of the Nrf2–Keap1 signaling pathway in cancer. Genes Dev 27(20):2179–2191. https://doi.org/10.1101/gad.225680.113
Niture SK, Kaspar JW, Shen J, Jaiswal AK (2010) Nrf2 signaling and cell survival. Toxicol Appl Pharmacol 244(1):37–42. https://doi.org/10.1016/j.taap.2009.06.009
Lau A, Villeneuve NF, Sun Z, Wong PK, Zhang DD (2008) Dual roles of Nrf2 in cancer. Pharmacol Res 58(5–6):262–270. https://doi.org/10.1016/j.phrs.2008.09.003
de la Vega MR, Chapman E, Zhang DD (2018) NRF2 and the hallmarks of cancer. Cancer Cell 34(1):21–43. https://doi.org/10.1016/j.ccell.2018.03.022
Singh A, Bodas M, Wakabayashi N, Bunz F, Biswal S (2010) Gain of Nrf2 function in non-small-cell lung cancer cells confers radioresistance. Antioxid Redox Signal 13(11):1627–1637. https://doi.org/10.1089/ars.2010.3219
Singh A, Boldin-Adamsky S, Thimmulappa RK, Rath SK, Ashush H, Coulter J, Blackford A, Goodman SN, Bunz F, Watson WH, Gabrielson E (2008) RNAi-mediated silencing of nuclear factor erythroid-2–related factor 2 gene expression in non–small cell lung cancer inhibits tumor growth and increases efficacy of chemotherapy. Can Res 68(19):7975–7984. https://doi.org/10.1158/0008-5472.can-08-1401
Zhang M, Zhang C, Zhang L, Yang Q, Zhou S, Wen Q, Wang J (2015) Nrf2 is a potential prognostic marker and promotes proliferation and invasion in human hepatocellular carcinoma. BMC Cancer 15(1):1–2. https://doi.org/10.1186/s12885-015-1541-1
Tong YH, Zhang B, Yan YY, Fan Y, Yu JW, Kong SS, Zhang D, Fang L, Su D, Lin NM (2017) Dual-negative expression of Nrf2 and NQO1 predicts superior outcomes in patients with non-small cell lung cancer. Oncotarget 8(28):45750. https://doi.org/10.18632/oncotarget.17403
Harvey CJ, Thimmulappa RK, Singh A, Blake DJ, Ling G, Wakabayashi N, Fujii J, Myers A, Biswal S (2009) Nrf2-regulated glutathione recycling independent of biosynthesis is critical for cell survival during oxidative stress. Free Radic Biol Med 46(4):443–453. https://doi.org/10.1016/j.freeradbiomed.2008.10.040
Oh ET, Park HJ (2015) Implications of NQO1 in cancer therapy. BMB Rep 48(11):609. https://doi.org/10.5483/BMBRep.2015.48.11.190
Takekuma M, Kuji S, Tanaka A, Takahashi N, Abe M, Hirashima Y (2015) Platinum sensitivity and non-cross-resistance of cisplatin analogue with cisplatin in recurrent cervical cancer. J Gynecol Oncol 26(3):185–192. https://doi.org/10.3802/jgo.2015.26.3.185
Zhu H, Luo H, Zhang W, Shen Z, Hu X, Zhu X (2016) Molecular mechanisms of cisplatin resistance in cervical cancer. Drug Des Dev Ther 10:1885. https://doi.org/10.2147/DDDT.S106412
Filippova M, Filippov V, Williams VM, Zhang K, Kokoza A, Bashkirova S, Duerksen-Hughes P (2014) Cellular levels of oxidative stress affect the response of cervical cancer cells to chemotherapeutic agents. BioMed Res Int. https://doi.org/10.1155/2014/574659
Kontostathi G, Zoidakis J, Makridakis M, Lygirou V, Mermelekas G, Papadopoulos T, Vougas K, Vlamis-Gardikas A, Drakakis P, Loutradis D, Vlahou A (2017) Cervical cancer cell line secretome highlights the roles of transforming growth factor-beta-induced protein ig-h3, peroxiredoxin-2, and NRF2 on cervical carcinogenesis. BioMed Res Int. https://doi.org/10.1155/2017/4180703
Cho JM, Manandhar S, Lee HR, Park HM, Kwak MK (2008) Role of the Nrf2-antioxidant system in cytotoxicity mediated by anticancer cisplatin: implication to cancer cell resistance. Cancer Lett 260(1–2):96–108. https://doi.org/10.1016/j.canlet.2007.10.022
Ma X, Zhang J, Liu S, Huang Y, Chen B, Wang D (2012) Nrf2 knockdown by shRNA inhibits tumor growth and increases efficacy of chemotherapy in cervical cancer. Cancer Chemother Pharmacol 69(2):485–494. https://doi.org/10.1007/s00280-011-1722-9
Itoh K, Mimura J, Yamamoto M (2010) Discovery of the negative regulator of Nrf2, Keap1: a historical overview. Antioxid Redox Signal 13(11):1665–1678. https://doi.org/10.1089/ars.2010.3222
Kim J, Keum YS (2016) NRF2, a key regulator of antioxidants with two faces towards cancer. Oxid Med Cell Longev. https://doi.org/10.1155/2016/2746457
Suzuki T, Yamamoto M (2015) Molecular basis of the Keap1–Nrf2 system. Free Radic Biol Med 88:93–100. https://doi.org/10.1016/j.freeradbiomed.2015.06.006
Jannatifar R, Parivar K, Roodbari NH, Nasr-Esfahani MH (2020) The effect of N-acetyl-cysteine on NRF2 antioxidant gene expression in asthenoteratozoospermia men: a clinical trial study. Int J Fertil Steril 14(3):171
Jacob Victorino V, Pizzatti L, Michelletti P, Panis C (2014) Oxidative stress, redox signaling and cancer chemoresistance: putting together the pieces of the puzzle. Curr Med Chem 21(28):3211–3226. https://doi.org/10.2174/0929867321666140601164647
Traverso N, Ricciarelli R, Nitti M, Marengo B, Furfaro AL, Pronzato MA, Marinari UM, Domenicotti C (2013) Role of glutathione in cancer progression and chemoresistance. Oxidative Med Cell Longev. https://doi.org/10.1155/2013/9729138
Ramesh PS, Devegowda DE, Naik PR, Doddamani PA, Nataraj SM (2018) Evaluating the feasibility of nested PCR as a screening tool to detect HPV infection in saliva of oral squamous cell carcinoma subjects. J Clin Diagnostic Res 1:12. https://doi.org/10.7860/JCDR/2020/44371.13825
Skehan P, Storeng R, Scudiero D, Monks A, McMahon J, Vistica D, Warren JT, Bokesch H, Kenney S, Boyd MR (1990) New colorimetric cytotoxicity assay for anticancer-drug screening. JNCI J Natl Cancer Inst 82(13):1107–1112. https://doi.org/10.1093/jnci/82.13.1107
Anantharaju PG, Reddy DB, Padukudru MA, Chitturi CM, Vimalambike MG, Madhunapantula SV (2017) Induction of colon and cervical cancer cell death by cinnamic acid derivatives is mediated through the inhibition of Histone Deacetylases (HDAC). PLoS ONE 12(11):e0186208. https://doi.org/10.1371/journal.pone.0186208
Schmittgen TD, Livak KJ (2008) Analyzing real-time PCR data by the comparative CT method. Nat Protoc 3(6):1101–1108. https://doi.org/10.1038/nprot.2008.73
Prochaska HJ, Santamaria AB (1988) Direct measurement of NAD (P) H: quinone reductase from cells cultured in microtiter wells: a screening assay for anticarcinogenic enzyme inducers. Anal Biochem 169(2):328–336. https://doi.org/10.1016/0003-2697(88)90292-8
Rahman I, Kode A, Biswas SK (2006) Assay for quantitative determination of glutathione and glutathione disulfide levels using enzymatic recycling method. Nat Protoc 1(6):3159–3165. https://doi.org/10.1038/nprot.2006.378
Shailasree S, Venkataramana M, Niranjana SR, Prakash HS (2015) Cytotoxic effect of p-coumaric acid on neuroblastoma, N2a cell via generation of reactive oxygen species leading to dysfunction of mitochondria inducing apoptosis and autophagy. Mol Neurobiol 51(1):119–130. https://doi.org/10.1007/s12035-014-8700-2
Acknowledgements
The authors would like to acknowledge the infrastructure support provided by the Department of Science & Technology to CEMR Laboratory (CR-FST-LS-1/2018/178) and to the Department of Biochemistry (SR/FST/LS-1-539/2012), the laboratory facilities provided by CEMR Laboratory, Department of Biochemistry, and JSS Academy of Higher Education & Research (Mysore, Karnataka, India). Pushkal Sinduvadi Ramesh (PSR) acknowledges the award of Senior Research Fellowship (08/721(0001)/2019-EMR-I) from Council of Scientific and Industrial Research (CSIR), Government of India.
Funding
No funding was received for conducting this study.
Author information
Authors and Affiliations
Contributions
Conceptualization: PSR and DD; Data curation, Formal analysis, Methodology: PSR, SR, SHU, and SC; Project administration, Resources and Supervision: SMN and DD; Writing-original draft: PSR; Writing-review & editing: SMN and DD; Final approval of the manuscript: PSR, SR, SHU, SC, SMN and DD.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest to declare that are relevant to the content of this article.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ramesh, P.S., Raja, S., Udayakumar, S.H. et al. Role of NRF2 cascade in determining the differential response of cervical cancer cells to anticancer drugs: an in vitro study. Mol Biol Rep 49, 109–119 (2022). https://doi.org/10.1007/s11033-021-06848-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-021-06848-2