Abstract
Cancer immunotherapy is a rapidly evolving concept that has been given the tag "fifth pillar" of cancer therapy while radiation therapy, chemotherapy, surgery and targeted therapy remain the other four pillars. This involves the stimulation of the immune system to control tumor growth and it specifically targets the neoplastic cells rather than the normal cells. Conventional chemotherapy has many limitations which include drug resistance, recurrence of cancer and severe adverse effects. Immunology has made major treatment breakthroughs for several cancers such as colorectal cancer, prostate cancer, breast cancer, lung cancer, liver cancer, kidney cancer, stomach cancer, acute lymphoblastic leukaemia etc. Currently, therapeutic strategies harnessing the immune system involve Checkpoint inhibitors, Chimeric antigen receptor T cells (CAR T cells), Monoclonal antibodies, Cancer vaccines, Cytokines, Radio-immunotherapy and Oncolytic virus therapy. The molecular characterization of several tumor antigens (TA) indicates that these TA can be utilized as promising candidates in cancer immunotherapy strategies. Here in this review, we highlight and summarize the different categories of emerging cancer immunotherapies along with the immunologically recognized tumor antigens involved in the tumor microenvironment.
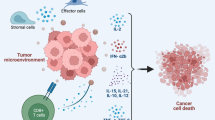
Graphic abstract



Similar content being viewed by others
Data availability
The authors confirm that the data supporting the findings of this research are available within the manuscript.
References
Freddie B, Ferlay J (2018) Global cancer statistics 2018:GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CAAC 2018(68):394–424
Qiao J, Liu Z, Fu YX (2016) Adapting conventional cancer treatment for immunotherapy. J Mol Med 94(5):489–495
Zitvogel L, Apetoh L, Ghiringhelli F, Kroemer G (2008) Immunological aspects of cancer chemotherapy. Nat Rev Immunol 8(1):59–73
Berkey FJ (2010) Managing the adverse effects of radiation therapy. Am Fam Phys 82(4):381–388
Baskar R, Lee KA, Yeo R, Yeoh KW (2012) Cancer and radiation therapy: current advances and future directions. Int J Med Sci 9(3):193
Stephan K, Matthias I, Sebastian K (2019) Advances in cancer immunotherapy 2019 – latest trends. Exp Clin Cancer Res 38(268):1–11
Sato H, Okonogi N, Nakano T (2020) Rationale of combination of anti-PD-1/PD-L1 antibody therapy and radiotherapy for cancer treatment. Int J Clin Oncol 25(5):801–809
Vermeulen K, Van Bockstaele DR, Berneman ZN (2003) The cell cycle: a review of regulation, deregulation and therapeutic targets in cancer. Cell Prolif 36(3):131–149
Demaria O, Cornen S, Daëron M, Morel Y, Medzhitov R, Vivier E (2019) Harnessing innate immunity in cancer therapy. Nature 574(7776):45–56
Mesri EA, Feitelson MA, Munger K (2014) Human viral oncogenesis: a cancer hallmarks analysis. Cell Host Microbe 15(3):266–282
Kambayashi T, Laufer TM (2014) Atypical MHC class II-expressing antigen-presenting cells: can anything replace a dendritic cell? Nat Rev Immunol 14(11):719–730
Wang S, He Z, Wang X, Li H, Liu XS (2019) Antigen presentation and tumor immunogenicity in cancer immunotherapy response prediction. Elife 8:e49020
De Charette M, Marabelle A, Houot R (2016) Turning tumor cells into antigen presenting cells: the next step to improve cancer immunotherapy? Eur J Cancer 68:134–147
Eggermont LJ, Paulis LE, Tel J, Figdor CG (2014) Towards efficient cancer immunotherapy: advances in developing artificial antigen-presenting cells. Trends Biotechnol 32(9):456–465
Smith CC, Selitsky SR, Chai S, Armistead PM, Vincent BG, Serody JS (2019) Alternative tumor-specific antigens. Nat Rev Cancer 19(8):465–478
Zhang JY (2004) Tumor-associated antigen arrays to enhance antibody detection for cancer diagnosis. Cancer Detect Prev 28(2):114–118
Fortner RT, Damms-Machado A, Kaaks R (2017) Systematic review: tumor-associated antigen autoantibodies and ovarian cancer early detection. Gynecol Oncol 147(2):465–480
Akashi T, Oimomi H, Nishiyama KI, Nakashima M, Arita Y, Sumii T, Kimura T, Ito T, Nawata H, Watanabe T (2003) Expression and diagnostic evaluation of the human tumor–associated antigen RCAS1 in pancreatic cancer. Pancreas 26(1):49–55
Casiano CA, Mediavilla-Varela M, Tan EM (2006) Tumor-associated antigen arrays for the serological diagnosis of cancer. Mol Cell Proteom 5(10):1745–1759
Ribas A, Hanson DC, Noe DA, Millham R, Guyot DJ, Bernstein SH, Canniff PC, Sharma A, Gomez-Navarro J (2007) Tremelimumab (CP-675,206), a cytotoxic T lymphocyte–associated antigen 4 blocking monoclonal antibody in clinical development for patients with cancer. Oncologist 12(7):873–883
Degroote H, Van Dierendonck A, Geerts A, Van Vlierberghe H, Devisscher L (2018) Preclinical and clinical therapeutic strategies affecting tumor-associated macrophages in hepatocellular carcinoma. J Immunol Res 2018:7819520
Pandya PH, Murray ME (2016) The immune system in cancer pathogenesis: potential therapeutic approaches. J Immunol Res 2016:4273943
McNamara MA, Nair SK, Holl EK (2015) RNA-based vaccines in cancer immunotherapy. J Immunol Res 2015:794528
Minati R, Perreault C, Thibault P (2020) A roadmap toward the definition of actionable tumor-specific antigens. Front Immunol 11:583287
Castle JC, Uduman M, Pabla S, Stein RB, Buell JS (2019) Mutation-derived neoantigens for cancer immunotherapy. Front Immunol 10:1856
Jiang T, Shi T, Zhang H, Hu J, Song Y, Wei J, Ren S, Zhou C (2019) Tumor neoantigens: from basic research to clinical applications. J Hematol Oncol 12(1):1–3
Lee S, Margolin K (2011) Cytokines in cancer immunotherapy. Cancers 3(4):3856–3893
Marshal HT, Djamgoz MBA (2018) Immuno-oncology: emerging targets and combination therapies. Front Oncol 8:1–29
Parayath N, Padmakumar S, Nair SV, Menon D, Amiji MM (2020) Strategies for targeting cancer immunotherapy through modulation of the tumor microenvironment. Regener Eng Transl Med 6(1):29–49
Albini A, Bruno A, Noonan DM, Mortara L (2018) Contribution to tumor angiogenesis from innate immune cells within the tumor microenvironment: implications for immunotherapy. Front Immunol 9:527
Syn NL, Teng MW, Mok TS, Soo RA (2017) De-novo and acquired resistance to immune checkpoint targeting. Lancet Oncol 18(12):e731–e741
Francisco LM, Sage PT, Sharpe AH (2010) The PD-1 pathway in tolerance and autoimmunity. Immunol Rev 236:219–242
Fife BT, Pauken KE (2011) The role of the PD-1 pathway in autoimmunity and peripheral tolerance. Ann NY Acad Sci 1217(1):45–59
Zitvogel L, Kroemer G (2012) Targeting PD-1/PD-L1 interactions for cancer immunotherapy. Oncoimmunology 1(8):1223–1225
Braun-Prado K, Petzl-Erler ML (2007) Programmed cell death 1 gene (PDCD1) polymorphism and pemphigus foliaceus (fogo selvagem) disease susceptibility. Genet Mol Biol 30(2):314–321
Ohigashi Y, Sho M, Yamada Y, Tsurui Y, Hamada K, Ikeda N, Mizuno T, Yoriki R, Kashizuka H, Yane K, Tsushima F (2005) Clinical significance of programmed death-1 ligand-1 and programmed death-1 ligand-2 expression in human esophageal cancer. Clin Cancer Res 11(8):2947–2953
Chemnitz JM, Parry RV, Nichols KE, June CH, Riley JL (2004) SHP-1 and SHP-2 associate with immunoreceptor tyrosine-based switch motif of programmed death 1 upon primary human T cell stimulation, but only receptor ligation prevents T cell activation. J Immunol 173(2):945–954
Jiao P, Geng Q, Jin P, Su G, Teng H, Dong J, Yan B (2018) Small molecules as PD-1/PD-L1 pathway modulators for cancer immunotherapy. Curr Pharm Des 24(41):4911–4920
Latchman Y, Wood CR, Chernova T, Chaudhary D, Borde M, Chernova I, Iwai Y, Long AJ, Brown JA, Nunes R, Greenfield EA (2001) PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat Immunol 2(3):261–268
Chen L (2004) Co-inhibitory molecules of the B7–CD28 family in the control of T-cell immunity. Nat Rev Immunol 4(5):336–347
Yang H, Zhou X, Sun L, Mao Y (2019) Correlation between PD-L2 expression and clinical outcome in solid cancer patients: a meta-analysis. Front Oncol 9:47
Rozali EN, Hato SV, Robinson BW, Lake RA, Lesterhuis WJ (2012) Programmed death ligand 2 in cancer-induced immune suppression. Clin Dev Immunol 2012:656340
Maverakis E, Kim K, Shimoda M, Gershwin ME, Patel F, Wilken R, Raychaudhuri S, Ruhaak LR, Lebrilla CB (2015) Glycans in the immune system and the altered glycan theory of autoimmunity: a critical review. J Autoimmunity 57:1–3
Akira S, Uematsu S, Takeuchi O (2006) Pathogen recognition and innate immunity. Cell 124(4):783–801
Van Duin D, Medzhitov R, Shaw AC (2006) Triggering TLR signaling in vaccination. Trends Immunol 27(1):49–55
Tsuji S, Matsumoto M, Takeuchi O, Akira S, Azuma I, Hayashi A, Toyoshima K, Seya T (2000) Maturation of human dendritic cells by cell wall skeleton of Mycobacterium bovis bacillus Calmette-Guerin: involvement of toll-like receptors. Infect Immunity 68(12):6883–6890
Creticos PS, Schroeder JT, Hamilton RG, Balcer-Whaley SL, Khattignavong AP, Lindblad R, Li H, Coffman R, Seyfert V, Eiden JJ, Broide D (2006) Immunotherapy with a ragweed–Toll-like receptor 9 agonist vaccine for allergic rhinitis. N Engl J Med 355(14):1445–1455
Kanzler H, Barrat FJ, Hessel EM, Coffman RL (2007) Therapeutic targeting of innate immunity with Toll-like receptor agonists and antagonists. Nat Med 13(5):552–559
Kanneganti TD, Lamkanfi M, Núñez G (2007) Intracellular NOD-like receptors in host defense and disease. Immunity 27(4):549–559
Saxena M, Yeretssian G (2014) NOD-like receptors: master regulators of inflammation and cancer. Front Immunol 5:327
Kondelkova K, Vokurková D, Krejsek J, Borská L, Fiala Z, Ctirad A (2010) Regulatory T cells (TREG) and their roles in immune system with respect to immunopathological disorders. Acta Medica 53:73–77
Mary LD (2014) Mechanism of action of immunotherapy. Semin Oncol 41:3–13
Davis BP, Ballas ZK (2017) Biologic response modifiers: indications, implications, and insights. J Allergy Clin Immunol 139(5):1445–1456
Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144(5):646–674
Knutson KL, Disis ML (2005) Tumor antigen-specific T helper cells in cancer immunity and immunotherapy. Cancer Immunol Immunother 54(8):721–728
Upadhyay S, Sharma N, Gupta KB, Dhiman M (2018) Role of immune system in tumor progression and carcinogenesis. J Cell Biochem 119(7):5028–5042
Weinberg RA, Hanahan D (2000) The hallmarks of cancer. Cell 100(1):57–70
Sauerborn M, Brinks V, Jiskoot W, Schellekens H (2010) Immunological mechanism underlying the immune response to recombinant human protein therapeutics. Trends Pharmacol Sci 31(2):53–59
Calderwood SK, Khaleque MA, Sawyer DB, Ciocca DR (2006) Heat shock proteins in cancer: chaperones of tumorigenesis. Trends Biochem Sci 31(3):164–172
Wang XY, Kaneko Y, Repasky E, Subjeck JR (2000) Heat shock proteins and cancer immunotherapy. Immunol Investig 29(2):131–137
Chatterjee S, Burns TF (2017) Targeting heat shock proteins in cancer: a promising therapeutic approach. Int J Mol Sci 18(9):1978
Das JK, Xiong X, Ren X, Yang JM, Song J (2019) Heat shock proteins in cancer immunotherapy. J Oncol. https://doi.org/10.1155/2019/3267207
Lanneau D, Brunet M, Frisan E, Solary E, Fontenay M, Garrido C (2008) Heat shock proteins: essential proteins for apoptosis regulation. J Cell Mol Med 12(3):743–761
Vidyasagar A, Wilson NA, Djamali A (2012) Heat shock protein 27 (HSP27): biomarker of disease and therapeutic target. Fibrogenesis Tissue Repair 5(1):1–7
Eftekharzadeh B, Banduseela VC, Chiesa G, Martínez-Cristóbal P, Rauch JN, Nath SR, Schwarz DM, Shao H, Marin-Argany M, Di Sanza C, Giorgetti E (2019) Hsp70 and Hsp40 inhibit an inter-domain interaction necessary for transcriptional activity in the androgen receptor. Nat Commun 10(1):1–4
Mayer MP, Bukau B (2005) Hsp70 chaperones: cellular functions and molecular mechanism. Cell Mol Life Sci 62(6):670–684
Moser C, Lang SA, Stoeltzing O (2009) Heat-shock protein 90 (Hsp90) as a molecular target for therapy of gastrointestinal cancer. Anticancer Res 29(6):2031–2042
Caudill MM, Li Z (2001) HSPPC-96: a personalised cancer vaccine. Expert Opin Biol Ther 1(3):539–547
Geng H, Zhang GM, Xiao H, Yuan Y, Li D, Zhang H, Qiu H, He YF, Feng ZH (2006) HSP70 vaccine in combination with gene therapy with plasmid DNA encoding sPD-1 overcomes immune resistance and suppresses the progression of pulmonary metastatic melanoma. Int Jn Cancer 118(11):2657–2664
Gong J, Zhu B, Murshid A, Adachi H, Song B, Lee A, Liu C, Calderwood SK (2009) T cell activation by heat shock protein 70 vaccine requires TLR signaling and scavenger receptor expressed by endothelial cells-1. J Immunol 183(5):3092–3098
Gong J, Zhang Y, Durfee J, Weng D, Liu C, Koido S, Song B, Apostolopoulos V, Calderwood SK (2010) A heat shock protein 70-based vaccine with enhanced immunogenicity for clinical use. J Immunol 184(1):488–496
Zong J, Wang C, Liu B, Liu M, Cao Y, Sun X, Yao Y, Sun G (2013) Human hsp70 and HPV16 oE7 fusion protein vaccine induces an effective antitumor efficacy. Oncol Rep 30(1):407–412
Klimczak M, Biecek P, Zylicz A, Zylicz M (2019) Heat shock proteins create a signature to predict the clinical outcome in breast cancer. Sci Rep 9(1):1–5
Kumar S, Principe DR, Singh SK, Viswakarma N, Sondarva G, Rana B, Rana A (2020) Mitogen-activated protein kinase inhibitors and t-cell-dependent immunotherapy in cancer. Pharmaceuticals 13(1):9
Murshid A, Gong J, Stevenson MA, Calderwood SK (2011) Heat shock proteins and cancer vaccines: developments in the past decade and chaperoning in the decade to come. Expert Rev Vaccines 10(11):1553–1568
Xiong D, Wang Y, Singavi AK, Mackinnon AC, George B, You M (2018) Immunogenomic landscape contributes to hyperprogressive disease after anti-PD-1 immunotherapy for cancer. iScience 9:258–277
Tournier C (2013) The 2 faces of JNK signaling in cancer. Genes Cancer 4(9–10):397–400
Roy CR, Mocarski ES (2007) Pathogen subversion of cell-intrinsic innate immunity. Nat Immunol 8(11):1179–1187
Arthur JS, Ley SC (2013) Mitogen-activated protein kinases in innate immunity. Nat Rev Immunol 13(9):679–692
Bhattacharyya ND, Feng CG (2020) Regulation of T helper cell fate by TCR signal strength. Front Immunol 11:624
Ohkusu-Tsukada K, Toda M, Udono H, Kawakami Y, Takahashi K (2010) Targeted inhibition of IL-10-secreting CD25− Treg via p38 MAPK suppression in cancer immunotherapy. Eur J Immunol 40(4):1011–1021
Romagnani S (2006) Regulation of the T cell response. Clin Exp Allergy 36(11):1357–1366
Boislève F, Kerdine-Römer S, Pallardy M (2005) Implication of the MAPK pathways in the maturation of human dendritic cells induced by nickel and TNF-α. Toxicology 206(2):233–244
Homet B, Ribas A (2014) New drug targets in metastatic melanoma. J Pathol 232(2):134–141
Shimizu Y, Suzuki T, Yoshikawa T, Endo I, Nakatsura T (2019) Next-generation cancer immunotherapy targeting glypican-3. Front Oncol 9:248
Ho M, Kim H (2011) Glypican-3: a new target for cancer immunotherapy. Eur J Cancer 47(3):333–338
Trinh TL, Puszyk W, Liu C (2014) Epigenetic regulation of glypican-3 in hepatocellular carcinoma. Cancer Res 74(19):418
Li L, Jin R, Zhang X, Lv F, Liu L, Liu D, Liu K, Li N, Chen D (2012) Oncogenic activation of glypican-3 by c-Myc in human hepatocellular carcinoma. Hepatology 56(4):1380–1390
Miller DM, Thomas SD, Islam A, Muench D, Sedoris K (2012) c-Myc and cancer metabolism. Clin Cancer Res 18(20):5546–5553
Shimizu Y, Suzuki T, Yoshikawa T, Tsuchiya N, Sawada Y, Endo I, Nakatsura T (2018) Cancer immunotherapy-targeted glypican-3 or neoantigens. Cancer Sci 109(3):531–541
Ratan C, Cicily KD, Nair B, Nath LR (2020) MUC glycoproteins: potential biomarkers and molecular targets for cancer therapy. Curr Cancer Drug Targets 21:132
Hollingsworth MA, Swanson BJ (2004) Mucins in cancer: protection and control of the cell surface. Nat Rev Cancer 4(1):45–60
Moncada DM, Kammanadiminti SJ, Chadee K (2003) Mucin and Toll-like receptors in host defense against intestinal parasites. Trends Parasitol 19(7):305–311
Mahla RS, Reddy CM, Prasad D, Kumar H (2013) Sweeten PAMPs: role of sugar complexed PAMPs in innate immunity and vaccine biology. Front Immunol 4:248
Ueno K, Koga T, Kato K, Golenbock DT, Gendler SJ, Kai H, Kim KC (2008) MUC1 mucin is a negative regulator of toll-like receptor signaling. Am J Respir Cell Mol Biol 38(3):263–268
Kotera Y, Fontenot JD, Pecher G, Metzgar RS, Finn OJ (1994) Humoral immunity against a tandem repeat epitope of human mucin MUC-1 in sera from breast, pancreatic, and colon cancer patients. Cancer Res 54(11):2856–2860
Shental-Bechor D, Levy Y (2008) Effect of glycosylation on protein folding: a close look at thermodynamic stabilization. Proc Natl Acad Sci 105(24):8256–8261
Taylor-Papadimitriou J, Burchell JM, Graham R, Beatson R (2018) Latest developments in MUC1 immunotherapy. Biochem Soc Trans 46(3):659–668
Roulois D, Grégoire M, Fonteneau JF (2013) MUC1-specific cytotoxic T lymphocytes in cancer therapy: induction and challenge. BioMed Res Int 2013:871936
Syrkina MS, Rubtsov MA (2019) MUC1 in cancer immunotherapy: new hope or phantom menace? Biochem Mosc 84(7):773–781
Collins NB, Al Abosy R, Miller B, Bi K, Manguso R, Yates K, Haining WN. PI3K activated tumors evade tumor immunity by promoting an inhibitory myeloid microenvironment
Groh V, Wu J, Yee C, Spies T (2002) Tumor-derived soluble MIC ligands impair expression of NKG2D and T-cell activation. Nature 419(6908):734–738
Nicolini A, Carpi A (2009) Immune manipulation of advanced breast cancer: an interpretative model of the relationship between immune system and tumor cell biology. Med Res Rev 29(3):436–471
Thomas MS, Mitchell JS, DeNucci CC, Martin AL, Shimizu Y (2008) The p110γ isoform of phosphatidylinositol 3-kinase regulates migration of effector CD4 T lymphocytes into peripheral inflammatory sites. J Leukoc Biol 84(3):814–823
Tang CH, Yamamoto A, Lin YT, Fong YC, Tan TW (2010) Involvement of matrix metalloproteinase-3 in CCL5/CCR5 pathway of chondrosarcomas metastasis. Biochem Pharmacol 79(2):209–217
O’Donnell JS, Massi D, Teng MW, Mandala M (2018) PI3K-AKT-mTOR inhibition in cancer immunotherapy, redux. In: Kim JB (ed) Seminars in cancer biology, vol 48. Academic Press, Cambridge, pp 91–103
Lin A, Yan WH (2015) Human leukocyte antigen-G (HLA-G) expression in cancers: roles in immune evasion, metastasis and target for therapy. Mol Med 21(1):782–791
Lin A, Chen HX, Zhu CC, Zhang X, Xu HH, Zhang JG, Wang Q, Zhou WJ, Yan WH (2010) Aberrant human leucocyte antigen-G expression and its clinical relevance in hepatocellular carcinoma. J Cell Mol Med 14(8):2162–2171
Wiendl H, Mitsdoerffer M, Hofmeister V, Wischhusen J, Bornemann A, Meyermann R, Weiss EH, Melms A, Weller M (2002) A functional role of HLA-G expression in human gliomas: an alternative strategy of immune escape. J Immunol 168(9):4772–4780
Agaugué S, Carosella ED, Rouas-Freiss N (2011) Role of HLA-G in tumor escape through expansion of myeloid-derived suppressor cells and cytokinic balance in favor of Th2 versus Th1/Th17. Blood 117(26):7021–7031
Lin A, Zhang X, Xu HH, Xu DP, Ruan YY, Yan WH (2012) HLA-G expression is associated with metastasis and poor survival in the Balb/c nu/nu murine tumor model with ovarian cancer. Int J Cancer 131(1):150–157
Zhang Y, Yu S, Han Y, Wang Y, Sun Y (2018) Human leukocyte antigen-G expression and polymorphisms promote cancer development and guide cancer diagnosis/treatment. Oncol Lett 15(1):699–709
Zeestraten EC, Reimers MS, Saadatmand S, Dekker JT, Liefers GJ, Van Den Elsen PJ, Van De Velde CJ, Kuppen PJ (2014) Combined analysis of HLA class I, HLA-E and HLA-G predicts prognosis in colon cancer patients. Br J Cancer 110(2):459–468
Brown DM, Ruoslahti E (2004) Metadherin, a cell surface protein in breast tumors that mediates lung metastasis. Cancer Cell 5(4):365–374
Chen X, Dong K, Long M, Lin F, Wang X, Wei J, Ren J, Zhang H (2012) Serum anti-AEG-1 auto-antibody is a potential novel biomarker for malignant tumors. Oncol Lett 4(2):319–323
Dhiman G, Srivastava N, Goyal M, Rakha E, Lothion-Roy J, Mongan NP, Miftakhova RR, Khaiboullina SF, Rizvanov AA, Baranwal M (2019) Metadherin: a therapeutic target in multiple cancers. Front Oncol 9:349
Lee SG, Su ZZ, Emdad L, Sarkar D, Franke TF, Fisher PB (2008) Astrocyte elevated gene-1 activates cell survival pathways through PI3K-Akt signaling. Oncogene 27(8):1114–1121
Emdad L, Lee SG, Su ZZ, Jeon HY, Boukerche H, Sarkar D, Fisher PB (2009) Astrocyte elevated gene-1 (AEG-1) functions as an oncogene and regulates angiogenesis. Proc Natl Acad Sci 106(50):21300–21305
Zhu GC, Yu CY, She L, Tan HL, Li G, Ren SL, Su ZW, Wei M, Huang DH, Tian YQ, Su RN (2015) Metadherin regulation of vascular endothelial growth factor expression is dependent upon the PI3K/Akt pathway in squamous cell carcinoma of the head and neck. Medicine 94(6):e502
Li PP, Feng LL, Chen N, Ge XL, Lv X, Lu K, Ding M, Yuan D, Wang X (2015) Metadherin contributes to the pathogenesis of chronic lymphocytic leukemia partially through Wnt/β-catenin pathway. Med Oncol 32(2):21
Yoo BK, Emdad L, Su ZZ, Villanueva A, Chiang DY, Mukhopadhyay ND, Mills AS, Waxman S, Fisher RA, Llovet JM, Fisher PB (2009) Astrocyte elevated gene-1 regulates hepatocellular carcinoma development and progression. J Clin Investig 119(3):465–477
Shi X, Wang X (2015) The role of MTDH/AEG-1 in the progression of cancer. Int J Clin Exp Med 8(4):4795
Zhao Y, Kong X, Li X, Yan S, Yuan C, Hu W, Yang Q (2011) Metadherin mediates lipopolysaccharide-induced migration and invasion of breast cancer cells. PLoS ONE 6(12):e29363
Abdel Ghafar MT, Gharib F, Abdel-Salam S, Elkhouly RA, Elshora A, Shalaby KH, El-Guindy D, El-Rashidy MA, Soliman NA, Abu-Elenin MM, Allam AA (2020) Role of serum Metadherin mRNA expression in the diagnosis and prediction of survival in patients with colorectal cancer. Mol Biol Rep 47(4):2509
Wang Z, Wei YB, Gao YL, Yan B, Yang JR, Guo Q (2014) Metadherin in prostate, bladder, and kidney cancer: a systematic review. Mol Clin Oncol 2(6):1139–1144
Dhiman G, Lohia N, Jain S, Baranwal M (2016) Metadherin peptides containing CD4+ and CD8+ T cell epitopes as a therapeutic vaccine candidate against cancer. Microbiol Immunol 60(9):646–652
Oh T, Ivan ME, Sun MZ, Safaee M, Fakurnejad S, Clark AJ, Sayegh ET, Bloch O, Parsa AT (2014) PI3K pathway inhibitors: potential prospects as adjuncts to vaccine immunotherapy for glioblastoma. Immunotherapy 6(6):737–753
Hongming Z, Jibei C (2018) Current status and future directions of cancer immunotherapy. JCA 9(10):1773–1781
Bonavita E, Bromley CP, Jonsson G, Pelly VS, Sahoo S, Walwyn-Brown K, Mensurado S, Moeini A, Flanagan E, Bell CR, Chiang SC (2020) Antagonistic inflammatory phenotypes dictate tumor fate and response to immune checkpoint blockade. Immunity 53(6):1215–1229
Martha MR, Estuardo AC, Augusto RM (2017) Immunotherapy and gene therapy as novel treatments for cancer. Colomb Med 48(3):138–147
Julian A, Marin A, Bhagirathbhai D, Aixa E (2018) Next generation of immune checkpoint therapy in cancer: new developments and challenges. J Hematol Oncol 11(39):1–20
Hashem O, Samaresh S, Rami A (2017) PD-1 and PD-L1 Checkpoint signaling inhibition for cancer immunotherapy: mechanism, combinations, and clinical outcome. Front Pharmacol 8:1–15
Garber K (2018) Driving T-cell Immunotherapy to solid tumors. Nat Biotechnol 36:215–219
Elizabeth IB, Anupam D (2016) CTLA-4 and PD-1 pathways similarities, differences, and implications of their inhibition. Am J Clin Oncol 39(1):98–106
Zahavi D, Weiner L (2020) Monoclonal antibodies in cancer therapy. Antibodies 9(3):34
Li S, Schmitz KR, Jeffrey PD, Wiltzius JJ, Kussie P, Ferguson KM (2005) Structural basis for inhibition of the epidermal growth factor receptor by cetuximab. Cancer Cell 7(4):301–311
Patel D, Bassi R, Hooper A, Prewett M, Hicklin DJ, Kang X (2009) Anti-epidermal growth factor receptor monoclonal antibody cetuximab inhibits EGFR/HER-2 heterodimerization and activation. Int J Oncol 34(1):25–32
Slamon DJ, Godolphin W, Jones LA, Holt JA, Wong SG, Keith DE, Levin WJ, Stuart SG, Udove J, Ullrich A (1989) Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science 244(4905):707–712
Chen JS, Lan K, Hung MC (2003) Strategies to target HER2/neu overexpression for cancer therapy. Drug Resist Updates 6(3):129–136
Wang J, Xu B (2019) Targeted therapeutic options and future perspectives for HER2-positive breast cancer. Signal Transduct Target Ther 4(1):1–22
Maloney DG, Grillo-López AJ, White CA, Bodkin D, Schilder RJ, Neidhart JA, Janakiraman N, Foon KA, Liles TM, Dallaire BK, Wey K (1997) IDEC-C2B8 (Rituximab) anti-CD20 monoclonal antibody therapy in patients with relapsed low-grade non-Hodgkin’s lymphoma. Blood 90(6):2188–2195
Anand R (2019) Combination of CTLA-4 and PD-1 blockers for treatment of cancer. J Exp Clin Cancer Res 38(255):1–12
Kimiz G, Gulce I, Biray AC (2018) Monoclonal antibodies in cancer immunotherapy. Mol Biol Rep 45(6):2935–2940
Miliotou AN, Papadopoulou LC (2018) CAR T-cell therapy: a new era in cancer immunotherapy. Curr Pharm Biotechnol 19(1):5–18
Darvin P, Toor SM, Nair S (2018) Immune checkpoint inhibitors: recent progress and potential biomarkers. Exp Mol Med 50(165):1–11
Xiuyan W, Isabelle R (2016) Clinical manufacturing of CAR T cells: foundation of a promising therapy. Mol Ther Oncol 3:1–7
Hammerstrom AE, Cauley DH, Atkinson BJ, Sharma P (2011) Cancer immunotherapy: sipuleucel-T and beyond. Pharmacotherapy 31(8):813–828
Lollini PL, Cavallo F, Nanni P, Quaglino E (2015) The promise of preventive cancer vaccines. Vaccines 3(2):467–489
Hu Z, Ott PA, Wu CJ (2018) Towards personalized, tumor-specific, therapeutic vaccines for cancer. Nat Rev Immunol 18(3):168–182
Van der Burg SH, Arens R, Ossendorp F, van Hall T, Melief CJ (2016) Vaccines for established cancer: overcoming the challenges posed by immune evasion. Nat Rev Cancer 16(4):219–233
Wong KK, Li WA, Mooney DJ, Dranoff G (2016) Advances in therapeutic cancer vaccines. Adv Immunol 130:191–249
Maeng H, Terabe M, Berzofsky JA (2018) Cancer vaccines: translation from mice to human clinical trials. Curr Opin Immunol 51:111–122
Finn OJ (2018) The dawn of vaccines for cancer prevention. Nat Rev Immunol 18(3):183–194
Gardner T, Elzey B, Hahn NM (2012) Sipuleucel-T (Provenge) autologous vaccine approved for treatment of men with asymptomatic or minimally symptomatic castrate-resistant metastatic prostate cancer. Hum Vaccines Immunother 8(4):534–539
Mougel A, Terme M, Tanchot C (2019) Therapeutic cancer vaccine and combinations with antiangiogenic therapies and immune checkpoint blockade. Front Immunol 10:467
Bose A, Taylor JL, Alber S, Watkins SC, Garcia JA, Rini BI, Ko JS, Cohen PA, Finke JH, Storkus WJ (2011) Sunitinib facilitates the activation and recruitment of therapeutic anti-tumor immunity in concert with specific vaccination. Int J Cancer 129(9):2158–2170
Oliver F, Nikhil V (2015) Immunotherapy for bladder cancer. Res Rep Urol 7:65–79
Alice M, Magali T, Corinne T (2019) Therapeutic cancer vaccine and combinations with antiangiogenic therapies and immune checkpoint blockade. Front Immunol 10:1–27
Berraondo P, Sanmamed MF, Ochoa MC (2019) Cytokines in clinical cancer immunotherapy. BJC 120:6–15
Sylvia L, Kim M (2011) Cytokines in cancer immunotherapy. Cancers 3(4):3856–3893
Zaheer J, Kim H, Lee YJ, Kim JS, Lim SM (2019) Combination radioimmunotherapy strategies for solid tumors. Int J Mol Sci 20(22):5579
Larson SM, Carrasquillo JA (2015) Radioimmunotherapy of human tumors. Nat Rev Cancer 15(6):347–360
Puvvada SD, Guillén-Rodríguez JM, Yan J, Inclán L, Heard K, Rivera XI, Anwer F, Mahadevan D, Schatz JH, Persky DO (2018) Yttrium-90-Ibritumomab tiuxetan (Zevalin®) radioimmunotherapy after cytoreduction with ESHAP chemotherapy in patients with relapsed follicular non-Hodgkin lymphoma: final results of a phase II study. Oncology 94(5):274–280
Di Maria Jiang AF, Nguyen LT, Neel BG, Sacher A, Rottapel R, Wang BX, Ohashi PS, Sridhar SS (2019) Phase I study of local radiation and tremelimumab in patients with inoperable locally recurrent or metastatic breast cancer. Oncotarget 10(31):2947
Bahig H, Aubin F, Stagg J, Gologan O, Ballivy O, Bissada E, Nguyen-Tan FP, Soulières D, Guertin L, Filion E, Christopoulos A (2019) Phase I/II trial of Durvalumab plus Tremelimumab and stereotactic body radiotherapy for metastatic head and neck carcinoma. BMC Cancer 19(1):1–5
Karam SD, Raben D (2019) Radioimmunotherapy for the treatment of head and neck cancer. Lancet Oncol 20(8):e404–e416
Corrò C, Dutoit V, Koessler T (2021) Emerging trends for radio-immunotherapy in rectal cancer. Cancers 13(6):1374
Bauerschmitz GJ, Kanerva A, Wang M, Herrmann I, Shaw DR, Strong TV, Desmond R, Rein DT, Dall P, Curiel DT, Hemminki A (2004) Evaluation of a selectively oncolytic adenovirus for local and systemic treatment of cervical cancer. Int J Cancer 111(2):303–309
Said D, Anne CA (2002) Cancer vaccines and immunotherapy. Br Med Bull 62:149–162
Junaid R, Johannes ML, Gettinger SN (2018) Oncolytic virus immunotherapy: future prospects for oncology. JITC 6(140):1–13
Kumar AR, Devan AR, Nair B, Nath LR (2021) Anti-VEGF mediated immunomodulatory role of phytochemicals: scientific exposition for plausible HCC treatment. Curr Drug Targets 22:1288
Emmett VS (2019) Developing combination strategies using PD-1 checkpoint inhibitors to treat cancer. Semin Immunopathol 41(1):21–30
Duaa OK, Heather JB, Silvia M (2019) Combining immune checkpoint inhibitors: established and emerging targets and strategies to improve outcomes in melanoma. Front Immunol 10:453
Yifan W, Weiye D, Li N (2018) Combining immunotherapy and radiotherapy for cancer treatment: current challenges and future directions. Front Pharmacol 9:185
Asna N, Livoff A, Batash R (2018) Radiation therapy and immunotherapy: a potential combination in cancer treatment. Curr Oncol 25(5):454–460
Ralph EV, Benjamin TC, Claire V (2014) Combinations of immunotherapy and radiation in cancer therapy. Front Oncol 4:325
Powles T, Balar A, Gravis G, Jones R, Ravaud A, Florence J, Grivas P, Petrylak DP, Galsky M, Carles J, Sridhar S (2019) An adaptive, biomarker directed platform study in metastatic urothelial cancer (BISCAY) with durvalumab in combination with targeted therapies. Ann Oncol 30:v356–v357
Shuji M (2019) Durvalumab for the treatment of non-small cell lung cancer. Expert Rev Anticancer Ther 19(12):1009–1016
Julie MC, James LG (2019) Product review: avelumab, an anti-PD-L1 antibody. Hum VaccinImmunother 15(4):891–908
Akintunde A, Zoaib R (2019) Immune checkpoint inhibitors of PD-L1 as cancer therapeutics. J Hematol Oncol 12(92):1–13
Wu X, Gu Z, Chen Y (2019) Application of PD-1 Blockade in Cancer Immunotherapy. Comput Struct Biotechnol J 17:661–674
Rusquec P, Calbiac O, Robert M, Campone M, Frenel JS (2019) Clinical utility of pembrolizumab in the management of advanced solid tumors: an evidence-based review on the emerging new data. Cancer Manag Res 11:4297–4312
Verma V, Sprave T, Haque W (2018) A systematic review of the cost and cost-effectiveness studies of immune checkpoint inhibitors. J Immunother Cancer 6(128):1–15
Qin S, Xu L, Yi M, Yu S (2019) Novel immune checkpoint targets: moving beyond PD-1 and CTLA-4. Mol Cancer 18(1):155
Marin-Acevedo JA, Dholaria B, Soyano AE, Knutson KL (2018) Next generation of immune checkpoint therapy in cancer: new developments and challenges. J Hematol Oncol 11(1):39
Jain MD, Bachmeier CA, Phuoc VH, Chavez JC (2018) Axicabtageneciloleucel (KTE-C19), an anti-CD19 CAR T therapy for the treatment of relapsed/refractory aggressive B-cell non-Hodgkin’s lymphoma. Ther Clin Risk Manag 14:1007–1017
Boyiadzis MM, Dhodapkar MV, Brentjens RJ (2018) Chimeric antigen receptor (CAR) T therapies for the treatment of hematologic malignancies: clinical perspective and significance. J Immunother Cancer 6(1):137
Kamat AM, Bellmunt J, Galsky MD (2017) Society for Immunotherapy of Cancer consensus statement on Immunotherapy for the treatment of bladder carcinoma. J Immunother Cancer 5(68):1–16
Riley RS, June CH, Langer R, Mitchell MJ (2019) Delivery technologies for cancer immunotherapy. Nat Rev Drug Discov 18(3):175–196
Amaria RN, Reuben A, Cooper ZA, Wargo JA (2015) Update on use of aldesleukin for treatment of high-risk metastatic melanoma. Immunotargets Ther 4:79–89
Asmana NR (2014) Human interferon alpha-2b: a therapeutic protein for cancer treatment. Scientifica (Cairo) 2014:1–9
Bubna AK (2015) Imiquimod - Its role in the treatment of cutaneous malignancies. Indian J Pharmacol 47(4):354–359
Ventola CL (2017) Cancer Immunotherapy, part 2: efficacy, safety, and other clinical considerations. Phar Ther 42(7):452–463
Coulson A, Levy A, Gossell WM (2014) Monoclonal antibodies in cancer therapy: mechanisms, successes and limitations. West Indian Med J 63(6):650–654
Kohlhapp FJ, Zloza A, Kaufman HL (2015) Talimogenelaherparepvec (T-VEC) as cancer immunotherapy. Drugs Today (Barc) 51(9):549–558
Newman MJ, Benani DJ (2016) A review of blinatumomab, a novel immunotherapy. J Oncol Pharm Pract 22(4):639–645
Acknowledgements
We acknowledge the support from Amrita Vishwa Vidyapeetham. We express our sincere gratitude to Dr Shanthikumar V Nair, Dean of research, AIMS and Dr Sabitha M, Principal, Amrita School of Pharmacy for all the facilities provided.
Funding
We acknowledge the support of the Amrita Vishwa Vidyapeetham SEED grant (Project ID: K-PHAR-20-627) to LRN. This review work was supported by a research grant from Amrita Vishwa Vidyapeetham under the PhD Student Research University Grant Scheme to ARK.
Author information
Authors and Affiliations
Contributions
LRN and BSV designed, conceptualized the review, revised and proofread the manuscript. ARK collected the kinds of literature and drafting of the first draft. ARD carried out a literature review and revised the draft. BLN carried out artwork.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kumar, A.R., Devan, A.R., Nair, B. et al. Harnessing the immune system against cancer: current immunotherapy approaches and therapeutic targets. Mol Biol Rep 48, 8075–8095 (2021). https://doi.org/10.1007/s11033-021-06752-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-021-06752-9