Abstract
Backgrounds
Epithelial mesenchymal transition (EMT) is a critical process involved in the invasion and metastasis of cancer, including lung cancer (LC). Transforming growth factor (TGF)-β is one of factors capable of inducing EMT. Polyinosinic-polycytidylic acid (polyI:C), a synthetic agonist for toll-like receptor (TLR) 3, can enhance immune responses and has been used as an adjuvant for cancer vaccines; however, it remains unclear whether it influences other process, such as EMT. In the present study, we examined the effects of polyI:C on TGF-β-treated A549 human LC cells.
Methods and results
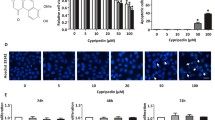
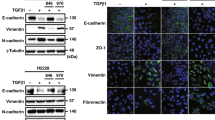
By in vitro cell proliferation assay, polyI:C showed no effect on the growth of A549 cells treated with TGF-β1 at the concentration range up to 10 μg/ml; however, it markedly suppressed the motility in a cell scratch and a cell invasion assay. By Western blotting, polyI:C dramatically decreased TGF-β1-induced Ak strain transforming (Akt) phosphorylation and increased phosphatase and tensin homologue (PTEN) expression without affecting the Son of mothers against decapentaplegic (Smad) 3 phosphorylation or the expression level of E-cadherin, N-cadherin or Snail, indicating that polyI:C suppressed cell motility independently of the ‘cadherin switching’. The Akt inhibitor perifosine inhibited TGF-β1-induced cell invasion, and the PTEN-specific inhibitor VO-OHpic appeared to reverse the inhibitory effect of polyI:C.
Conclusion
PolyI:C has a novel function to suppress the motility of LC cells undergoing EMT by targeting the phosphatidylinositol 3-kinase/Akt pathway partly via PTEN and may prevent or reduce the metastasis of LC cells.





Similar content being viewed by others
References
Dela Cruz CS, Tanoue LT, Matthay RA (2011) Lung cancer: epidemiology, etiology, and prevention. Clin Chest Med 32:605–644
Lemjabbar-Alaoui H, Hassan OU, Yang YW, Buchanan P (2015) Lung cancer: biology and treatment options. Biochim Biophys Acta 1856:189–210
Siegel RL, Miller KD, Fuchs HE, Jemal A (2021) Cancer statistics, 2021. CA Cancer J Clin 71:7–33
Xie S, Wu Z, Qi Y, Wu B, Zhu X (2021) The metastasizing mechanisms of lung cancer: recent advances and therapeutic challenges. Biomed Pharmacother 138:111450
Menju T, Date H (2021) Lung cancer and epithelial-mesenchymal transition. Gen Thorac Cardiovasc Surg 69:781–789
Roberts AB, Thompson NL, Heine U, Flanders C, Sporn MB (1988) Transforming growth factor-beta: possible roles in carcinogenesis. Br J Cancer 57:594–600
Lamouille S, Derynck R (2007) Cell size and invasion in TGF-beta-induced epithelial to mesenchymal transition is regulated by activation of the mTOR pathway. J Cell Biol 178:437–451
Hamidi A, Song J, Thakur N et al (2017) TGF-β promotes PI3K-AKT signaling and prostate cancer cell migration through the TRAF6-mediated ubiquitylation of p85α. Sci Signal 10:eaa14186
Lee JH, Chinnathambi A, Alharbi SA, Shair OHM, Sethi G, Ahn KS (2019) Farnesol abrogates epithelial to mesenchymal transition process through regulating Akt/mTOR pathway. Pharmacol Res 150:104504
Moses HL, Roberts AB, Derynck R (2016) The discovery and early days of TGF-β: a historical perspective. Cold Spring Harb Perspect Biol 88:a021865
Forte G, Rega A, Morello S et al (2012) Polyinosinic-polycytidylic acid limits tumor outgrowth in a mouse model of metastatic lung cancer. J Immunol 188:5357–5364
Urban-Wojciuk Z, Khan MM, Oyler BL et al (2019) The role of TLRs in anti-cancer immunity and tumor rejection. Front Immunol 10:2388
Gnjatic S, Sawhney NB, Bhardwaj N (2010) Toll-like receptor agonists: are they good adjuvants. Cancer J 16:382–391
Salazar AM, Erlich RB, Mark A, Bhardwaj N, Herberman RB (2014) Therapeutic in situ autovaccination against solid cancers with intratumoral poly-ICLC: case report, hypothesis, and clinical trial. Cancer Immunol Res 2:720–724
Jiang Q, Wei H, Tian Z (2008) Poly I:C enhances cycloheximide-induced apoptosis of tumor cells through TLR3 pathway. BMC Cancer 8:12
Lau WH, Zhu XG, Ho SWT, Chang SC, Ding JL (2017) Combinatorial treatment with polyI:C and anti-IL6 enhances apoptosis and suppresses metastasis of lung cancer cells. Oncotarget 8:32884–32904
Guo Z, Chen L, Zhu Y et al (2012) Double-stranded RNA-induced TLR3 activation inhibits angiogenesis and triggers apoptosis of human hepatocellular carcinoma cells. Oncol Rep 27:396–402
Ma B, Herzog EL, Moore M et al (2016) RIG-like helicase regulation of chitinase 3-like 1 axis and pulmonary metastasis. Sci Rep 6:26299
Tissari J, Sirén J, Meri S, Julkunen I, Matikainen S (2005) IFN-alpha enhances TLR3-mediated antiviral cytokine expression in human endothelial and epithelial cells by up-regulating TLR3 expression. J Immunol 174:4289–4294
Lieber M, Smith B, Szakal A, Nelson-Rees W, Todaro G (1976) A continuous tumor-cell line from a human lung carcinoma with properties of type II alveolar epithelial cells. Int J Cancer 17:62–70
Schneider CA, Rasband WS, Eliceiri KW (2012) NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9:671–675
Liang CC, Park AY, Guan JL (2007) In vitro scratch assay: a convenient and inexpensive method for analysis of cell migration in vitro. Nat Protoc 2:329–333
Repesh LA (1989) A new in vitro assay for quantitating tumor cell invasion. Invasion Metastasis 9:192–208
Wang Y, Shi J, Chai K, Ying X, Zhou BP (2013) The role of snail in EMT and tumorigenesis. Curr Cancer Drug Targets 13:963–972
Kondapaka SB, Singh SS, Dasmahapatra GP, Sausville EA, Roy KK (2003) Perifosine, a novel alkylphospholipid, inhibits protein kinase B activation. Mol Cancer Ther 2:1093–1103
Govender D, Chetty R (2012) Gene of the month: PTEN. J Clin Pathol 65:601–603
Rosivatz E, Matthews JG, McDonald NQ et al (2006) A small molecule inhibitor for phosphatase and tensin homologue deleted on chromosome 10 (PTEN). ACS Chem Biol 1:780–790
Zhu X, Shao ZH, Li C et al (2014) TAT-protein blockade during ischemia/reperfusion reveals critical role for p85 PI3K-PTEN interaction in cardiomyocyte injury. PLoS One 9:e95622
Varthaman A, Moreau HD, Maurin M, Benaroch P (2016) TLR3-induced maturation of murine dendritic cells regulates CTL responses by modulating PD-L1 trafficking. PLoS One 11:e0167057
Xu J, Lamouille S, Derynck R (2009) TGF-beta-induced epithelial to mesenchymal transition. Cell Res 19:156–172
Georgescu MM (2010) PTEN tumor suppressor network in PI3K-Akt pathway control. Genes Cancer 1:1170–1177
Leslie NR, Yang X, Downes CP, Weijer CJ (2005) The regulation of cell migration by PTEN. Biochem Soc Trans 33:1507–1508
Yucel G, Oro AE (2011) Cell migration: GSK3β steers the cytoskeleton’s tip. Cell 144:319–321
Huang L, Zhang C, Su L, Song Z (2017) GSK3β attenuates TGF-β1 induced epithelial-mesenchymal transition and metabolic alterations in ARPE-19 cells. Biochem Biophys Res Commun 486:744–751
Matsumoto M, Kikkawa S, Kohase M, Miyake K, Seya T (2002) Establishment of a monoclonal antibody against human toll-like receptor 3 that blocks double-stranded RNA-mediated signaling. Biochem Biophys Res Commun 293:1364–1369
Acknowledgements
We are grateful to the members of the Department of Pathology and Experimental Medicine, Okayama University, for their useful discussion.
Funding
This study was funded by the Department of Pathology and Experimental Medicine, Okayama University.
Author information
Authors and Affiliations
Contributions
Conceptualization: TY; Formal analysis and investigation: TY, GT; Writing—TY, TY; Writing—review and editing: TY, TO, MF, AM; Funding acquisition: MF, TO, AM.
Corresponding author
Ethics declarations
Conflicts of interest
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yamaguchi, T., Yoshimura, T., Ohara, T. et al. PolyI:C suppresses TGF-β1-induced Akt phosphorylation and reduces the motility of A549 lung carcinoma cells. Mol Biol Rep 48, 6313–6321 (2021). https://doi.org/10.1007/s11033-021-06625-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-021-06625-1