Abstract
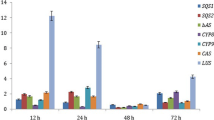
Saffron stigmas are widely used as food additives and as traditional medicine in Iran and many other countries. The unique taste, flavor and pharmaceutical properties of saffron stigmas are due to the presence of three apocarotenoids secondary metabolites crocin, picrocrocin and safranal. There is limited knowledge about the effect of environmental stresses on the metabolism of apocarotenoids in saffron. We analyzed the content of crocin and picrocrocin and the expression of key genes of apocarotenoid biosynthesis pathways (CsCCD2, CsCCD4, CsUGT2, CsCHY-β and CsLCYB) in saffron plants exposed to moderate (90 mM) and high (150 mM) salt (NaCl) concentrations. Measuring ion concentrations in leaves showed an increased accumulation of Na+ and decreased uptake of K+ in salt treated compared to control plants indicating an effective salt stress. HPLC analysis of apocarotenoids revealed that crocin production was significantly halted (P < 0.05) with increasing salt concentration while picrocrocin level did not change with moderate salt but significantly dropped by high salt concentration. Real-time PCR analysis revealed a progressive decrease in transcript levels of CsUGT2 and CsLCYB genes with increasing salt concentration (P < 0.05). The expression of CsCCD2 and CsCHY-β tolerated moderate salt concentration but significantly downregulated with high salt concentration. CsCCD4 however responded differently to salt concentration being decreased with moderate salt but increased at higher salt concentration. Our result suggested that salt stress had an adverse effect on the production of saffron apocarotenoids and it is likely influencing the quality of saffron stigma produced.



Similar content being viewed by others
References
Hasegawa PM, Bressan RA, Zhu JK, Bohnert HJ (2000) Plant cellular and molecular responses to high salinity. Annu Rev Plant Physiol Plant Mol Biol 51(1):463–499. https://doi.org/10.1146/annurev.arplant.51.1.463
Gill SS, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem 48(12):909–930. https://doi.org/10.1016/j.plaphy.2010.08.016
Zhang H, Han B, Wang T, Chen S, Li H, Zhang Y, Dai S (2012) Mechanisms of plant salt response: insights from proteomics. J Proteome Res 11(1):49–67. https://doi.org/10.1021/pr200861w
Pang C-H, Wang B-S (2008) Oxidative Stress and salt tolerance in plants. Progress Bot. https://doi.org/10.1007/978-3-540-72954-9_9
Ramakrishna A, Ravishankar GA (2011) Influence of abiotic stress signals on secondary metabolites in plants. Plant Signal Behav 6(11):1720–1731. https://doi.org/10.4161/psb.6.11.17613
Verma N, Shukla S (2015) Impact of various factors responsible for fluctuation in plant secondary metabolites. J Appl Res Med Aromat Plants 2(4):105–113. https://doi.org/10.1016/j.jarmap.2015.09.002
Yang L, Wen KS, Ruan X, Zhao YX, Wei F, Wang Q (2018) Response of plant secondary metabolites to environmental factors. Molecules. https://doi.org/10.3390/molecules23040762
Munns R (2002) Comparative physiology of salt and water stress. Plant Cell Environ 25(2):239–250. https://doi.org/10.1046/j.0016-8025.2001.00808.x
Dixon RA, Paiva NL (1995) Stress-induced phenylpropanoid metabolism. Plant Cell 7(7):1085–1097. https://doi.org/10.1105/tpc.7.7.1085
Waśkiewicz A, Muzolf-Panek M, Goliński P (2013) Phenolic content changes in plants under salt stress. In: Ecophysiology and responses of plants under salt stress. pp 283–314. doi:https://doi.org/10.1007/978-1-4614-4747-4_11
Wang JW, Wu JY (2013) Effective elicitors and process strategies for enhancement of secondary metabolite production in hairy root cultures. In: Biotechnology of hairy root systems. Advances in Biochemical Engineering/Biotechnology. pp 55–89. doi:https://doi.org/10.1007/10_2013_183
Halder M, Sarkar S, Jha S (2019) Elicitation: a biotechnological tool for enhanced production of secondary metabolites in hairy root cultures. Eng Life Sci 19(12):880–895. https://doi.org/10.1002/elsc.201900058
Yeats TH, Nagel R (2018) Subcellular spice trade routes: crocin biosynthesis in the saffron crocus (Crocus sativus). Plant Physiol 177(3):869–870. https://doi.org/10.1104/pp.18.00662
Auldridge ME, McCarty DR, Klee HJ (2006) Plant carotenoid cleavage oxygenases and their apocarotenoid products. Curr Opin Plant Biol 9(3):315–321
Nisar N, Li L, Lu S, Khin NC, Pogson BJ (2015) Carotenoid metabolism in plants. Mol Plant 8(1):68–82. https://doi.org/10.1016/j.molp.2014.12.007
Zhang M, Leng P, Zhang G, Li X (2009) Cloning and functional analysis of 9-cis-epoxycarotenoid dioxygenase (NCED) genes encoding a key enzyme during abscisic acid biosynthesis from peach and grape fruits. J Plant Physiol 166(12):1241–1252. https://doi.org/10.1016/j.jplph.2009.01.013
Escribano J, Alonso GL, Coca-Prados M, Fernandez JA (1996) Crocin, safranal and picrocrocin from saffron (Crocus sativus L.) inhibit the growth of human cancer cells in vitro. Cancer Lett 100:23–30. https://doi.org/10.1016/0304-3835(95)04067-6
Bukhari SI, Manzoor M, Dhar MK (2018) A comprehensive review of the pharmacological potential of Crocus sativus and its bioactive apocarotenoids. Biomed Pharmacother 98:733–745. https://doi.org/10.1016/j.biopha.2017.12.090
Huang FC, Horvath G, Molnar P, Turcsi E, Deli J, Schrader J, Sandmann G, Schmidt H, Schwab W (2009) Substrate promiscuity of RdCCD1, a carotenoid cleavage oxygenase from Rosa damascena. Phytochemistry 70(4):457–464. https://doi.org/10.1016/j.phytochem.2009.01.020
Molina R, Valero M, Navarro Y, Guardiola J, Garcia-Luis A (2005) Temperature effects on flower formation in saffron (Crocus sativus L.). Sci Horticult 103(3):361–379
Kabiri M, Rezadoost H, Ghassempour A (2017) A comparative quality study of saffron constituents through HPLC and HPTLC methods followed by isolation of crocins and picrocrocin. LWT 84:1–9. https://doi.org/10.1016/j.lwt.2017.05.033
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25(4):402–408. https://doi.org/10.1006/meth.2001.1262
Ahrazem O, Rubio-Moraga A, Argandoña-Picazo J, Castillo R, Gómez-Gómez L (2016) Intron retention and rhythmic diel pattern regulation of carotenoid cleavage dioxygenase 2 during crocetin biosynthesis in saffron. Plant Mol Biol 91(3):355–374. https://doi.org/10.1007/s11103-016-0473-8
Frusciante S, Diretto G, Bruno M, Ferrante P, Pietrella M, Prado-Cabrero A, Rubio-Moraga A, Beyer P, Gomez-Gomez L, Al-Babili S, Giuliano G (2014) Novel carotenoid cleavage dioxygenase catalyzes the first dedicated step in saffron crocin biosynthesis. Proc Natl Acad Sci USA 111(33):12246–12251. https://doi.org/10.1073/pnas.1404629111
Rajaei SM, Niknam V, Seyedi SM, Ebrahimzadeh H, Razavi K (2009) Contractile roots are the most sensitive organ in Crocus sativus to salt stress. Biol Plant 53(3):523–529. https://doi.org/10.1007/s10535-009-0095-y
Sepaskhah AR, Yarami N (2015) Interaction effects of irrigation regime and salinity on flower yield and growth of saffron. J Hortic Sci Biotechnol 84(2):216–222. https://doi.org/10.1080/14620316.2009.11512507
Mzabri I, Legsayer M, Aliyat F, Maldani M, Kouddane NE, Boukroute A, Bekkouch I, Berrichi A (2017) Effect of drought stress on the growth and development of saffron (Crocus Sativus L.) in eastern morocco. Atlas J Biol 20:364–370
Torbaghan ME, Ahmadi MM (2011) The effect of salt stress on flower yield and growth parameters of saffron (Crocus sativus L.) in greenhouse condition. Acta Hort 1184:55–62
Tarantilis PA, Tsoupras G, Polissiou M (1995) Determination of saffron (Crocus sativus L.) components in crude plant extract using high-performance liquid chromatography-UV-visible photodiode-array detection-mass spectrometry. J Chromatogr A 699(1–2):107–118. https://doi.org/10.1016/0021-9673(95)00044-n
Sánchez AM, Winterhalter P (2013) Carotenoid cleavage products in saffron (Crocus sativus L.). In: Carotenoid cleavage products. ACS Symposium Series. pp 45–63. doi:https://doi.org/10.1021/bk-2013-1134.ch005
Lage M, Cantrell CL (2009) Quantification of saffron (Crocus sativus L.) metabolites crocins, picrocrocin and safranal for quality determination of the spice grown under different environmental Moroccan conditions. Sci Horticult 121(3):366–373. https://doi.org/10.1016/j.scienta.2009.02.017
Zhou G, Li L, Lu J, Li J, Yao C, Sun P, Liu K, Dong Y, Qin L, Qian X (2020) Flower cultivation regimes affect apocarotenoid accumulation and gene expression during the development of saffron stigma. Hortic Environ Biotechnol 61(3):473–484. https://doi.org/10.1007/s13580-020-00248-4
Yarami N, Sepaskhah AR (2016) Effect of irrigation water salinity, manure application and planting method on qualitative compounds of saffron (Crocus sativus L.). Int J Plant Product 10(2):123–137. https://doi.org/10.22069/ijpp.2016.2784
Nambara E, Marion-Poll A (2005) Abscisic acid biosynthesis and catabolism. Annu Rev Plant Biol 56:165–185. https://doi.org/10.1146/annurev.arplant.56.032604.144046
Tuteja N (2007) Abscisic acid and abiotic stress signaling. Plant Signal Behav 2(3):135–138. https://doi.org/10.4161/psb.2.3.4156
Tuan PA, Kim JK, Lee S, Chae SC, Park SU (2013) Molecular characterization of carotenoid cleavage dioxygenases and the effect of gibberellin, abscisic acid, and sodium chloride on the expression of genes involved in the carotenoid biosynthetic pathway and carotenoid accumulation in the callus of Scutellaria baicalensis Georgi. J Agric Food Chem 61(23):5565–5572. https://doi.org/10.1021/jf401401w
Ahrazem O, Argandoña J, Fiore A, Rujas A, Rubio-Moraga Á, Castillo R, Gómez-Gómez L (2019) Multi-species transcriptome analyses for the regulation of crocins biosynthesis in Crocus. BMC Genomics. https://doi.org/10.1186/s12864-019-5666-5
Cazzonelli CI, Pogson BJ (2010) Source to sink: regulation of carotenoid biosynthesis in plants. Trends Plant Sci 15(5):266–274. https://doi.org/10.1016/j.tplants.2010.02.003
Dufresne C, Cormier F, Dorion S (1997) In vitro formation of crocetin glucosyl esters by Crocus sativus callus extract. Planta Med 63(2):150–153. https://doi.org/10.1055/s-2006-957633
Moraga AR, Nohales PF, Perez JA, Gomez-Gomez L (2004) Glucosylation of the saffron apocarotenoid crocetin by a glucosyltransferase isolated from Crocus sativus stigmas. Planta 219(6):955–966. https://doi.org/10.1007/s00425-004-1299-1
Rubio A, Rambla JL, Santaella M, Gómez MD, Orzaez D, Granell A, Gómez-Gómez L (2008) Cytosolic and plastoglobule-targeted carotenoid dioxygenases from Crocus sativus are both involved in β-ionone release. J Biol Chem 283(36):24816–24825
Baba SA, Mohiuddin T, Basu S, Swarnkar MK, Malik AH, Wani ZA, Abbas N, Singh AK, Ashraf N (2015) Comprehensive transcriptome analysis of Crocus sativus for discovery and expression of genes involved in apocarotenoid biosynthesis. BMC Genomics 16(1):698. https://doi.org/10.1186/s12864-015-1894-5
Rubio-Moraga A, Rambla JL, Fernandez-de-Carmen A, Trapero-Mozos A, Ahrazem O, Orzaez D, Granell A, Gomez-Gomez L (2014) New target carotenoids for CCD4 enzymes are revealed with the characterization of a novel stress-induced carotenoid cleavage dioxygenase gene from Crocus sativus. Plant Mol Biol 86(4–5):555–569. https://doi.org/10.1007/s11103-014-0250-5
Ohmiya A (2009) Carotenoid cleavage dioxygenases and their apocarotenoid products in plants. Plant Biotechnol 26(4):351–358
Babaei S, Niknam V, Behmanesh M (2019) Nitric oxide induced carotenoid contents in Crocus sativus under salinity. Nat Prod Res. https://doi.org/10.1080/14786419.2019.1608544
Ann BM, Devesh S, Gothandam K (2011) Effect of salt stress on expression of carotenoid pathway genes in tomato. J Stress Physiol Biochem 7(3):56–89
Cheng WS, Zeng DL, Zhang N, Zeng HX, Ren J, Shi XF, Li YH, Sun YH (2015) Expression analysis of salt stress on carotenoid pathway genes in watermelon. Adv Mater Res, 1073. Trans Tech Publ,
Maurya VK, Srinvasan R, Ramesh N, Anbalagan M, Gothandam K (2015) Expression of carotenoid pathway genes in three capsicum varieties under salt stress. Asian J Crop Sci 7(4):286–294
Sankari M, Hridya H, Sneha P, Doss CGP, Christopher JG, Mathew J, Zayed H, Ramamoorthy S (2019) Implication of salt stress induces changes in pigment production, antioxidant enzyme activity, and qRT-PCR expression of genes involved in the biosynthetic pathway of Bixa orellana L. Funct Integr Genomics 19(4):565–574. https://doi.org/10.1007/s10142-019-00654-7
Kim SH, Ahn YO, Ahn MJ, Lee HS, Kwak SS (2012) Down-regulation of beta-carotene hydroxylase increases beta-carotene and total carotenoids enhancing salt stress tolerance in transgenic cultured cells of sweetpotato. Phytochemistry 74:69–78. https://doi.org/10.1016/j.phytochem.2011.11.003
Acknowledgements
This research was founded by a grant provided by the Encyclopedia Research Center, Institute for Humanities and Cultural Studies.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors Declare no competing financial or non-financial interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Moslemi, F.S., Vaziri, A., Sharifi, G. et al. The effect of salt stress on the production of apocarotenoids and the expression of genes related to their biosynthesis in saffron. Mol Biol Rep 48, 1707–1715 (2021). https://doi.org/10.1007/s11033-021-06219-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-021-06219-x