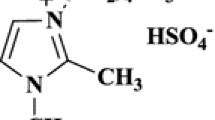
Abstract
An efficient and green strategy for the regioselective synthesis of highly functionalized pyranopyrazole via one-pot condensation of 3-methyl-1-phenyl-5-pyrazolone or EAA and hydrazine hydrate, substituted aromatic aldehydes with NMSM [(E)-N-Methyl-1-(methylthio)-2-nitro-ethenamine] in the existence of IL [(EMIM)Ac] as catalyst with solvent-free condition (SFC) is described. This domino protocol produces biologically substantial heterocycles through Knoevenagel condensation proceeded by Michael addition and O-cyclization with an eradication of methanethiol group, which create the one stereo-center and creation of “C–C, C–N, C–O, C=C, C=N, bonds.” The final product is produced by exceptionally easy filtering after the reaction mass was triturated with ethanol. The strategy's noteworthy features include the use of biodegradable IL catalyst, excellent to exceptional yield with rapid reaction times, applicability to a wide range of substrate, clear reaction profile, and straightforward workup process.
Graphical abstract








Similar content being viewed by others
Notes
aIsolated yield
References
Zuin VG, Eilks I, Elschami M, Kümmerer K (2021) Education in green chemistry and in sustainable chemistry: perspectives towards sustainability. Green Chem 23(4):1594–1608. https://doi.org/10.1039/D0GC03313H
Katariya AP, Bhagat DS, Katariya MV, Pawar RP (2017) Green techniques in organic synthesis and its advantages. J Med Chem Drug Discov 3:62–66
Jiang B, Rajale T, Wever W, Tu SJ, Li G (2010) Multicomponent reactions for the synthesis of heterocycles. Chem Asian J 5(11):2318–2335. https://doi.org/10.1002/asia.201000310
Naresh G, Lakkaniga NR, Kharbanda A, Yan W, Frett B (2019) Li HY (2019) Use of Imidazo [1, 2-a] pyridine as a carbonyl surrogate in a Mannich-Like, catalyst free, one-pot reaction. Eur J Org Chem 4:770–777. https://doi.org/10.1002/ejoc.201801430
Naresh G, Kharbanda A, Lakkaniga NR, Zhang L, Cooper R, Li HY, Frett B (2018) Catalyst free, C-3 functionalization of imidazo [1, 2-a] pyridines to rapidly access new chemical space for drug discovery efforts. Chem Commun 54(92):12954–12957. https://doi.org/10.1021/ol502072k
Naresh G, Kant R, Narender T (2014) Copper (II) catalyzed expeditious synthesis of furoquinoxalines through a one-pot three-component coupling strategy. Org Lett 16(17):4528–4531. https://doi.org/10.1021/ol502072k
Rotstein BH, Zaretsky S, Rai V, Yudin AK (2014) Small heterocycles in multicomponent reactions. Chem Rev 114(16):8323–8359. https://doi.org/10.1021/cr400615v
Boukis AC, Reiter K, Frölich M, Hofheinz D, Meier MAR (2018) Multicomponent reactions provide key molecules for secret communication. Nat Commun 9(1):1439. https://doi.org/10.1038/s41467-018-03784-x
Martins MAP, Frizzo CP, Moreira DN, Buriol L, Machado P (2009) Solvent-free heterocyclic synthesis. Chem Rev 109(9):4140–4182. https://doi.org/10.1021/cr9001098
Zangade S, Patil P (2019) A review on solvent-free methods in organic synthesis. Curr Org Chem 23(21):2295–2318. https://doi.org/10.2174/1385272823666191016165532
Metzger JO (1998) Solvent-free organic syntheses. Angew Chem Int Ed 37(21):2975–2978. https://doi.org/10.1002/(SICI)1521-3773(19981116)37:21%3c2975::AID-ANIE2975%3e3.0.CO;2-A
Olivier-Bourbigou H, Magna L, Morvan D (2010) Ionic liquids and catalysis: recent progress from knowledge to applications. Appl Catal Gen 373(1–2):1–56. https://doi.org/10.1016/j.apcata.2009.10.008
Shi F, Gu Y, Zhang Q, Deng Y (2004) Development of ionic liquids as green reaction media and catalysts. Catal Surv from Asia 8(3):179–186
Zhang Q, Zhang S, Deng Y (2011) Recent advances in ionic liquid catalysis. Green Chem 13(10):2619–2637. https://doi.org/10.1039/c1gc15334j
Das D, Banerjee R, Mitra A (2014) Bioactive and pharmacologically important pyrano[2,3-c]pyrazoles. J Chem Pharm Res 6(11):108–116
Mohamed NR, Khaireldin NY, Fahmy AF, El-Sayed AA (2010) Facile synthesis of fused nitrogen containing heterocycles as anticancer agents. Pharma Chem 2(1):400–417
Foloppe N, Fisher LM, Howes R, Potter A, Robertson AGS, Surgenor AE (2006) Identification of chemically diverse Chk1 inhibitors by receptor-based virtual screening. Bioorg Med Chem 14:4792–4802
Ramtekkar R, Kumarvel K, Vasuki G, Sekar K, Krishna R (2009) Computer-aided drug design of pyranopyrazoles and related compounds for checkpoint kinase-. Lett Drug Des Discovery 6:579–584
Saundane AR, Walmik P, Yarlakatti M, Katkar V, Verma VA (2014) Synthesis and biological activities of some new annulated pyrazolopyranopyrimidines and their derivatives containing indole nucleus: synthesis and biological activities of some new annulated pyrazolopyranopyrimidines and their derivatives containing indole nucleus. J Heterocycl Chem 51(2):303–314. https://doi.org/10.1002/jhet.1582
Ismail MMF, Khalifa NM, Fahmy HH, Nossier ES, Abdulla MM (2014) Design, docking, and synthesis of some new pyrazoline and pyranopyrazole derivatives as anti-inflammatory agents. J Heterocycl Chem 51(2):450–458. https://doi.org/10.1002/jhet.1757
Ueda T, Mase H, Oda N, Ito I (1891) Synthesis of pyrazolone derivatives XXXIX. Synthesis and analgesic activity of pyrano [2, 3-c] pyrazoles. Chem Pharm Bull (Tokyo) 29(12):3522–3528. https://doi.org/10.1248/cpb.29.3522
Capodanno D, Ferreiro JL, Angiolillo DJ (2013) Antiplatelet therapy: new pharmacological agents and changing paradigms. J Thromb Haemost 11:316–329. https://doi.org/10.1111/jth.12219
Tacconi G, Gatti G, Desimoni G, Messori VA (1980) New route to 4H-pyrano[2,3-c]pyrazoles. J Für Prakt Chem 322(5):831–834. https://doi.org/10.1002/prac.19803220519
Abdelrazek FM, Metz P, Metwally NH, El-Mahrouky SF (2006) Synthesis and molluscicidal activity of new cinnoline and pyrano [2,3-c]pyrazole derivatives. Arch Pharm (Weinheim) 339(8):456–460. https://doi.org/10.1002/ardp.200600057
Yadav DK, Quraishi MA (2012) Electrochemical investigation of substituted pyranopyrazoles adsorption on mild steel in acid solution. Ind Eng Chem Res 51(24):8194–8210. https://doi.org/10.1021/ie3002155
Vasuki G, Kumaravel K (2008) Rapid four-component reactions in water: synthesis of pyranopyrazoles. Tetrahedron Lett 49(39):5636–5638. https://doi.org/10.1016/j.tetlet.2008.07.055
Kanagaraj K, Pitchumani K (2010) Solvent-free multicomponent synthesis of pyranopyrazoles: Per-6-Amino-β-Cyclodextrin as a remarkable catalyst and host. Tetrahedron Lett 51(25):3312–3316. https://doi.org/10.1016/j.tetlet.2010.04.087
Zolfigol MA, Tavasoli M, Moosavi-Zare AR, Moosavi P, Kruger HG, Shiri M, Khakyzadeh V (2013) Synthesis of pyranopyrazoles using isonicotinic acid as a dual and biological organocatalyst. RSC Adv 3(48):25681. https://doi.org/10.1039/c3ra45289a
Reddy MBM, Jayashankara VP, Pasha MA (2010) Glycine-catalyzed efficient synthesis of pyranopyrazoles via one-pot multicomponent reaction. Synth Commun 40(19):2930–2934. https://doi.org/10.1080/00397910903340686
Katariya AP, Deshmukh SU, Munde SB, Katariya MV, Pawar RP (2019) Green and expeditious one pot synthesis of pyrano[2,3-c]pyrazole using potassium ter-butoxide catalyst in aqueous medium. Int J Green Herb Chem. https://doi.org/10.24214/IJGHC/GC/8/3/79097
Kanchithalaivan S, Sivakumar S, Ranjith Kumar R, Elumalai P, Ahmed QN, Padala AK (2013) Four-component domino strategy for the combinatorial synthesis of novel 1,4-Dihydropyrano[2,3- c ]Pyrazol-6-Amines. ACS Comb Sci 15(12):631–638. https://doi.org/10.1021/co4000997
Jayabal K, Paramasivan TP (2014) An expedient four-component domino protocol for the regioselective synthesis of highly functionalized pyranopyrazoles and chromenopyrazoles via nitroketene-N, S-acetal chemistry under solvent-free condition. Tetrahedron Lett 55(12):2010–2014. https://doi.org/10.1016/j.tetlet.2014.02.019
Survase DN, Chavan HV, Dongare SB, Ganapure SD, Helavi VB (2017) Indium chloride (InCl3) catalysed domino protocol for the regioselective synthesis of highly functionalized pyranopyrazoles under mild conditions. Iran Chem Commun 5:105–114
Khan MdM, Shareef S, Saigal S, Sahoo SCA (2019) Catalyst and solvent-free protocol for the sustainable synthesis of fused 4H-pyran derivatives. RSC Adv 9(45):26393–26401. https://doi.org/10.1039/C9RA04370E
Katariya AP, Yadav AR, Pawar OB, Pisal PM, Sangshetti JN, Katariya MV, Deshmukh SU (2022) An Efficient and green synthesis of tetrahydrobenzo[b]pyan derivatives using [(EMIM)Ac] at room temperature. ChemistrySelect. https://doi.org/10.1002/slct.202104184
Katariya AP, Katariya MV, Sangshetti J, Deshmukh SU (2022) Ionic liquid [(EMIM)Ac] catalyzed green and efficient synthesis of Pyrano[2,3- c ]Pyrazole derivatives. Polycycl Aromat Compd. https://doi.org/10.1080/10406638.2022.2077775
Katariya AP, Gaikwad PB, Kadam GG, Katariya MV, Deshmukh SU (2022) Ionic liquid promoted regio-selective synthesis of 2-Methyl Amino-3-Nitro-pyrano[3,2- c ]chromen-5-ones. ChemistrySelect. https://doi.org/10.1002/slct.202201295
Katariya AP, Deshmukh SU, Tekale SU, Katariya MV, Pawar RP (2021) Amberlite IR-120 catalyzed green and efficient one-pot synthesis of benzylpyrazolyl coumarin in aqueous medium. Lett Appl NanoBioSci 10(3):2525–2534. https://doi.org/10.33263/LIANBS103.25252534
Deshmukh SU, Sangshetti JN, Bhosale SV, Pawar RP (2021) Benzopyranyl phosphonate and β-phosphono malonates derivatives: an exciting breakthrough in chemistry. ChemistrySelect 6(4):617–629. https://doi.org/10.1002/slct.202004159
Deshmukh SU, Kharat KR, Kadam GG, Pawar RP (2018) Synthesis of novel α-aminophosphonate derivatives, biological evaluation as potent antiproliferative agents and molecular docking. ChemistrySelect 3(20):5552–5558. https://doi.org/10.1002/slct.201800798
Thorat VV, Dake SA, Deshmukh SU, RasokkiyamUddin EMF, Pawar RP (2013) Ionic liquid mediated synthesis of novel tetrahydroimidazo [1,2- a]pyrimidine-6-carboxylate derivatives. Lett Org Chem 10(3):178–184
Ansari SA, Deshmukh SU, Patil RB, Damale MG, Patil RH, Alkahtani HM, Almehizia AA, Al-Tuwajiri HM, Aleanizy FS, Alqahtani FY, Pathan SK, Sangshetti JN (2019) Identification of promising biofilm inhibitory and cytotoxic quinazolin-4-one derivatives: synthesis, evaluation molecular docking and ADMET studies. ChemistrySelect 4(12):3559–3566. https://doi.org/10.1002/slct.201803795
Acknowledgements
SAIF-PU, Panjab University, Chandigarh, provided the spectrum data, such as 1H & 13C NMR, to the authors, and they would like to express their gratitude for their assistance in this study. This research work would not have been possible without the general facilities provided by Deogiri College, Aurangabad.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
11030_2022_10572_MOESM1_ESM.docx
Supplementary file1 (DOCX 3321 KB) The IR, 1H, 13C NMR, and HRMS spectra of new and a few previously known pyranopyrazole compounds are included in a supporting information also consist of a table shows synthesized compound’s yield and melting point
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Katariya, A.P., Shirsath, P.D., Narode, H. et al. Unraveling the access to the regioselective synthesis of highly functionalized pyranopyrazoles using an ionic liquid catalyst. Mol Divers 27, 2633–2649 (2023). https://doi.org/10.1007/s11030-022-10572-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11030-022-10572-9