Abstract
The versatility of nitroaliphatics is demonstrated by using it in the syntheses of artemisinin derived dimers. A few novel artemisinin derived dimer and monomer have been synthesized using nitroalkane as linker.
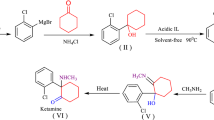
Graphical Abstract
Similar content being viewed by others
References
Seebach D, Colvin E, Lehr F, Weller T (1979) Nitroaliphatic compounds—ideal intermediates in organic synthesis?. Chimia 33: 1–18
Yoshikoshi A, Miyashita M (1985) Oxoalkylation of carbonyl compounds with conjugated nitroolefins. Acc Chem Res 18: 284–290. doi:10.1021/ar00117a005
Barrett AGM, Graboski GG (1986) Conjugated nitroalkenes: versatile intermediates in organic synthesis. Chem Rev 86: 751–762. doi:10.1021/cr00075a002
Verma RS, Kabalka GW (1986) Nitroalkanes in the synthesis of heterocyclic compounds. Heterocycles 24: 2645–2677
Rosini G, Ballini R (1988) Functionalized nitroalkanes as useful reagents for alkyl anion synthons. Synthesis 11: 833–847. doi:10.1055/s-1988-27726
Bachman GB, Hokama T (1959) Preparation of α-nitroketones, C-acylation of primary nitroparaffins. J Am Chem Soc 81: 4882–4885. doi:10.1021/ja01527a031
Baker DC, Dutt SR (1978) C-acylation of nitromethane. A synthetic route to α- nitroketones. Synthesis 6: 478
White EH, Considine WJ (1958) The acylation of salts of secondary nitroparaffins. J Am Chem Soc 80: 626–630. doi:10.1021/ja01536a031
Klayman D (1985) Qinghaosu (artemisini): an antimalarial drug from China. Science 228: 1049–1055. doi:10.1126/science.3887571
Chaturvedi D, Goswami A, Saikia PP, Barua NC, Rao PG (2010) Artemisin and its derivatives: a novel class of antimalarial and anticancer agents. Chem Soc Rev 39: 435–454. doi:10.1039/b816679j
Jung M, Li X, Bustos DA, Elsohly HN, McChesney JD (1989) A short and stereospecific synthesis of (+)-deoxoartemisinin and (−)-deoxodesoxyartemisinin. Tetrahedron Lett 30: 5973–5976. doi:10.1016/S0040-4039(01)93831-6
Jung M, Li X, Bustos DA, Elsohly HN, McChesney JD, Mihous WK (1990) Synthesis and antimalaraial activity of (+)-deoxoartemisinin. J Med Chem 33: 1516–1518. doi:10.1021/jm00167a036
Posner GH, Ploypradith P, Parker MH, O’Dowd H, Woo S-H, Northrop J, Krasavin M, Dolan P, Kensler TW, Xie S, Shapiro TA (1999) Antimalaraial, antiproliferative and antitumour activities of artemisinin derived chemically robust artemisinin dimmers. J Med Chem 42: 4275–4280. doi:10.1021/jm990363d
Ekthawatchai S, Kamchonwongpaisan S, Kongsaeree P, Tarnchompoo B, Thebtaranonth Y, Yuthavong Y (2001) C-16 artemisinin derivatives and their antimalarial and cytotoxic activities: syntheses of artemisinin monomers, dimers, trimers and tetramers by nucleophilic additions to artemisitene. J Med Chem 44: 4688–4695. doi:10.1021/jm0103007
Kalita D, Khan AT, Barua NC, Bez G (1999) Total synthesis of R-(+)-patulolide A and R-(−) -patulolide B: The macrolides isolated from Penicillium urticae mutant. Tetrahedron 55: 5177–5184. doi:10.1016/S0040-4020(99)00164-7
Kalita B, Barua NC, Bezbarua MS, Bez G (2001) Synthesis of 2-nitroalcohols by regioselective ring opening of epoxides with MgSO4/MeOH/NaNO2 system: a short synthesis of immunosuppressive agent FTY-720. Synlett 1411–1414. doi:10.1055/s-2001-16776
Borah JC, Gogoi S, Boruwa J, Kalita B, Barua NC (2004) A highly efficient synthesis of the C-13 side-chain of taxol using Shibasaki’s asymmetric Henry reaction. Tetrahedron Lett 45: 3689– 3691. doi:10.1016/j.tetlet.2004.02.150
Gogoi N, Boruwa J, Barua NC (2005) A total synthesis of (−)-bestatin using Shibasaki’s asymmetric Henry reaction. Tetrahedron Lett 46: 7581–7582. doi:10.1016/j.tetlet.2005.08.153
Boruwa J, Barua NC (2006) Stereoselective total synthesis of (+)-boronolide. Tetrahedron 62: 1193–1198. doi:10.1016/j.tet.2005.10.070
Gogoi N, Boruwa J, Barua NC (2006) A concise total synthesis of antifungal antibiotic (+)-preussin. Eur J Org Chem 2006: 1722–1725. doi:10.1002/ejoc.200500833
Saikia PP, Baishya G, Goswami A, Barua NC (2008) An efficient reduction protocol for the synthesis of β-hydroxycarbamates from β-nitro alcohols in one pot: a facile synthesis of (−)-β-conhydrine. Tetrahedron Lett 49: 6508–6511. doi:10.1016/j.tetlet.2008.08.113
Goswami A, Saikia PP, Barua NC, Bordoloi MJ, Yadav A, Bora TC, Gogoi BK, Saxena AK, Suri N, Sharma M (2010) Bio- transformation of artemisinin using soil microbe: direct C-acetoxylation of artemisinin at C-9 by Penicillium simplissimum. Bioorg Med Chem Lett 20: 359–361. doi:10.1016/j.bmcl.2009.10.097
Ballini R, Barboni L, Giarlo G (2004) The first conversion of primary alkyl halides to nitroalkanes under aqueous medium. J Org Chem 69: 6907–6908. doi:10.1021/jo049048b
Bergbreiter DE, Lalonde JJ (1987) Michael additions of nitroalkanes to α, β-unsaturated carbonyl compounds using KF/basic alumina. J Org Chem 52: 1601–1603. doi:10.1021/jo00384a040
Saikia AK, Hazarika MJ, Barua NC, Bezbarua MS, Sharma RP, Ghosh AC (1996) Direct synthesis of keto nitroaliphatics via retro-Henry reaction of cyclic 2-nitroalcohols by anhydrous copper sulfate adsorbed on silica gel. A short synthesis of (±)-phoracantholide I. Synthesis 981–985. doi:10.1055/s-1996-4318
Bezbarua MS, Saikia AK, Barua NC, Kalita D (1996) A short nantioselective formal synthesis of methyl (S)-(−)-6,8-dihydroxyoctanoate: a key intermediate for the synthesis of R-(+)-α-lipoic acid. Synthesis 1289–1290. doi:10.1055/s-1996-4380
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Goswami, A., Saikia, P.P., Saikia, B. et al. Dinitroaliphatics as linkers: application in the synthesis of novel artemisinin carba-dimer. Mol Divers 15, 707–712 (2011). https://doi.org/10.1007/s11030-010-9296-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11030-010-9296-8