Abstract
Diabetes mellitus is a chronic metabolic disorder and has been associated with cognitive dysfunction. In our earlier study, chronic Urtica dioica (UD) treatment significantly ameliorated diabetes induced associative and spatial memory deficit in mice. The present study was designed to explore the effect of UD leaves extract on muscarinic cholinergic system, which has long been known to be involved in cognition. Streptozotocin (STZ) (50 mg/kg, i.p., consecutively for 5 days) was used to induce diabetes followed by treatment with UD extract (50 mg/kg, oral) or rosiglitazone (5 mg/kg, oral) for 8 weeks. STZ-induced diabetic mice showed significant reduction in hippocampal muscarinic acetylcholine receptor-1 and choline acetyltransferase expressions. Chronic diabetes significantly up-regulated the protein expression of acetylcholinesterase associated with oxidative stress in hippocampus. Besides, STZ-induced diabetic mice showed hypolocomotion with up-regulation of muscarinic acetylcholine receptor-4 expression in striatum. Chronic UD treatment significantly attenuated the cholinergic dysfunction and oxidative stress in the hippocampus of diabetic mice. UD had no effect on locomotor activity and muscarinic acetylcholine receptor-4 expression in striatum. In conclusion, UD leaves extract has potential to reverse diabetes mediated alteration in muscarinic cholinergic system in hippocampus and thereby improve memory functions.






Similar content being viewed by others
References
Abdalla FH, Schmatz R, Cardoso AM, Carvalho FB, Baldissarelli J, de Oliveira JS, Rosa MM, Gonçalves Nunes MA, Rubin MA, da Cruz IB, Barbisan F, Dressler VL, Pereira LB, Schetinger MR, Morsch VM, Gonçalves JF, Mazzanti CM (2014) Quercetin protects the impairment of memory and anxiogenic-like behavior in rats exposed to cadmium: possible involvement of the acetylcholinesterase and Na(+), K(+)-ATPase activities. Physiol Behav 135:152–167
Ahangarpour A, Mohammadian M, Dianat M (2012) Antidiabetic effect of hydroalcholic urtica dioica leaf extract in male rats with fructose-induced insulin resistance. Iran J Med Sci 37:181–186
Alipour M, Salehi I, Ghadiri Soufi F (2012) Effect of exercise on diabetes-induced oxidative stress in the rat hippocampus. Iran Red Crescent Med J 14:222–228
Ambrogi-Lorenzini CG, Baldi E, Bucherelli C, Sacchetti B, Tassoni G (1999) Neural topography and chronology of memory consolidation: a review of functional inactivation findings. Neurobiol Learn Mem 71:1–18
Anisuzzaman AS, Uwada J, Masuoka T, Yoshiki H, Nishio M, Ikegaya Y, Takahashi N, Matsuki N, Fujibayashi Y, Yonekura Y, Momiyama T, Muramatsu I (2013) Novel contribution of cell surface and intracellular M1-muscarinic acetylcholine receptors to synaptic plasticity in hippocampus. J Neurochem 126:360–371
Beckmann DV, Carvalho FB, Mazzanti CM, Dos Santos RP, Andrades AO, Aiello G, Rippilinger A, Graça DL, Abdalla FH, Oliveira LS, Gutierres JM, Schetinger MR, Mazzanti A (2014) Neuroprotective role of quercetin in locomotor activities and cholinergic neurotransmission in rats experimentally demyelinated with ethidium bromide. Life Sci 103:79–87
Bhutada P, Mundhada Y, Bansod K, Bhutada C, Tawari S, Dixit P, Mundhada D (2010) Ameliorative effect of quercetin on memory dysfunction in streptozotocin-induced diabetic rats. Neurobiol Learn Mem 94:293–302
Bubser M, Byun N, Wood MR, Jones CK (2012) Handbook of experimental pharmacology. Springer, Berlin
Claiborne A (1985) Handbook of methods for oxygen radical research. CRC Press, Florida
Danaei G, Finucane MM, Lu Y, Singh GM, Cowan MJ, Paciorek CJ, Lin JK, Farzadfar F, Khang YH, Stevens GA, Rao M, Ali MK, Riley LM, Robinson CA, Ezzati M (2011) National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2.7 million participants. Lancet 378:31–40
Drever BD, Riedel G, Platt B (2011) The cholinergic system and hippocampal plasticity. Behav Brain Res 221:505–514
Ejaz Ahmed M, Khan MM, Javed H, Vaibhav K, Khan A, Tabassum R, Ashafaq M, Islam F, Safhi MM, Islam F (2013) Amelioration of cognitive impairment and neurodegeneration by catechin hydrate in rat model of streptozotocin-induced experimental dementia of Alzheimer’s type. Neurochem Int 62:492–501
Exner M, Hermann M, Hofbauer R, Kapiotis S, Speiser W, Held I, Seelos C, Gmeiner BM (2000) The salicylate metabolite gentisic acid, but not the parent drug, inhibits glucose autoxidation-mediated atherogenic modification of low density lipoprotein. FEBS Lett 470:47–50
Fazeli SA, Gharravi AM, Ghafari S, Jahanshahi M, Golalipour MJ (2008) The granule cell density of the dentate gyrus following administration of Urtica dioica extract to young diabetic rats. Folia Morphol (Warsz) 67:196–204
Gomeza J, Zhang L, Kostenis E, Felder C, Bymaster F, Brodkin J, Shannon H, Xia B, Deng C, Wess J (1999) Enhancement of D1 dopamine receptor-mediated locomotor stimulation in M(4) muscarinic acetylcholine receptor knockout mice. Proc Natl Acad Sci U S A 96:10483–10488
Hölscher C (1995) Inhibitors of cyclooxygenases produce amnesia for a passive avoidance task in the chick. Eur J Neurosci 7:1360–1365
Hornick A, Lieb A, Vo NP, Rollinger JM, Stuppner H, Prast H (2011) The coumarin scopoletin potentiates acetylcholine release from synaptosomes, amplifies hippocampal long-term potentiation and ameliorates anticholinergic- and age-impaired memory. Neuroscience 197:280–292
Hunter A, Roberts F (1988) The effect of pirenzepine on spatial learning in the Morris water maze. Pharmacol Biochem Behav 30:519–523
Khandelwal KR (2008) Practical pharmacognosy. Nirali prakashan, Pune
Kianbakht S, Khalighi-Sigaroodi F, Dabaghian FH (2013) Improved glycemic control in patients with advanced type 2 diabetes mellitus taking Urtica dioica leaf extract: a randomized double-blind placebo-controlled clinical trial. Clin Lab 59:1071–1076
Kou ZZ, Li CY, Hu JC, Yin JB, Zhang DL, Liao YH, Wu ZY, Ding T, Qu J, Li H, Li YQ (2014) Alterations in the neural circuits from peripheral afferents to the spinal cord: possible implications for diabetic polyneuropathy in streptozotocin-induced type 1 diabetic rats. Front Neural Circ 8:6
Kumar A, Rinwa P, Dhar H (2014) Possible nitric oxide modulation in the protective effects of rutin against experimental head trauma-induced cognitive deficits: behavioral, biochemical, and molecular correlates. J Surg Res 188:268–279
Lee CR, Shin EJ, Kim HC, Choi YS, Shin T, Wie MB (2011) Esculetin inhibits N-methyl-D-aspartate neurotoxicity via glutathione preservation in primary cortical cultures. Lab Anim Res 27:259–263
Lupien SB, Bluhm EJ, Ishii DN (2003) Systemic insulin-like growth factor-I administration prevents cognitive impairment in diabetic rats, and brain IGF regulates learning/memory in normal adult rats. J Neurosci Res 74:512–523
Lustman PJ, Griffith LS, Clouse RE (1988) Depression in adults with diabetes: results of 5-year follow-up study. Diabetes Care 11:605–612
Mahesh R, Kumar B, Jindal A, Bhatt S, Devadoss T, Pandey DK (2012) Antidepressant-like activity of (4-phenylpiperazin-1-yl) (quinoxalin-2-yl) methanone (4a), a novel 5-HT(3) receptor antagonist: an investigation in behaviour-based rodent models of depression. Indian J Pharmacol 44:560–565
More AS, Kumari RR, Gupta G, Kathirvel K, Lonare MK, Dhayagude RS, Kumar D, Kumar D, Sharma AK, Tandan SK (2012) Effect of S-methylisothiourea in acetaminophen-induced hepatotoxicity in rat. Naunyn Schmiedeberg’s Arch Pharmacol 385:1127–1139
Namazi N, Tarighat A, Bahrami A (2012) The effect of hydro alcoholic nettle (Urtica dioica) extract on oxidative stress in patients with type 2 diabetes: a randomized double-blind clinical trial. Pak J Biol Sci 15:98–102
Nouwen A, Nefs G, Caramlau I, Connock M, Winkley K, Lloyd CE, Peyrot M, Pouwer F (2011) Prevalence of depression in individuals with impaired glucose metabolism or undiagnosed diabetes: a systematic review and meta-analysis of the European Depression in Diabetes (EDID) Research Consortium. Diabetes Care 34:752–762
O’Reilly JA, Lynch M (2012) Rosiglitazone improves spatial memory and decreases insoluble Aβ(1–42) in APP/PS1 mice. J Neuroimmune Pharm 7:140–144
Oda Y (1999) Choline acetyltransferase: the structure, distribution and pathologic changes in the central nervous system. Pathol Int 49:921–937
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358
Orcic D, Franciskovic M, Bekvalac K, Svircev E, Beara I, Lesjak M, Mimica-Dukic N (2014) Quantitative determination of plant phenolics in Urtica dioica extracts by high-performance liquid chromatography coupled with tandem mass spectrometric detection. Food Chem 143:48–53
Panda S, Kar A (2006) Evaluation of the antithyroid, antioxidative and antihyperglycemic activity of scopoletin from Aegle marmelos leaves in hyperthyroid rats. Phytother Res 20:1103–1105
Patel SS, Udayabanu M (2013) Effect of Urtica dioica on memory dysfunction and hypoalgesia in an experimental model of diabetic neuropathy. Neurosci Lett 552:114–119
Patel SS, Udayabanu M (2014) Urtica dioica extract attenuates depressive like behavior and associative memory dysfunction in dexamethasone induced diabetic mice. Metab Brain Dis 29:121–130
Pipatpiboon N, Pratchayasakul W, Chattipakorn N, Chattipakorn SC (2012) PPARγ agonist improves neuronal insulin receptor function in hippocampus and brain mitochondria function in rats with insulin resistance induced by long term high-fat diets. Endocrinology 153:329–338
Potapenko ES, Biancardi VC, Zhou Y, Stern JE (2012) Altered astrocyte glutamate transporter regulation of hypothalamic neurosecretory neurons in heart failure rats. Am J Physiol Regul Integr Comp Physiol 303:R291–R300
Prabakaran D, Ashokkumar N (2013) Protective effect of esculetin on hyperglycemia-mediated oxidative damage in the hepatic and renal tissues of experimental diabetic rats. Biochimie 95:366–373
Prickaerts J, Blokland A, Honig W, Meng F, Jolles J (1995) Spatial discrimination learning and choline acetyltransferase activity in streptozotocin-treated rats: effects of chronic treatment with acetyl-L-carnitine. Brain Res 674:142–146
Qujeq D, Tatar M, Feizi F, Parsian H, Sohan Faraji A, Halalkhor S (2013) Effect of Urtica dioica leaf alcoholic and aqueous extracts on the number and the diameter of the islets in diabetic rats. Int J Mol Cell Med 2:21–26
Rinwa P, Kumar A (2014) Modulation of nitrergic signaling pathway by American ginseng attenuates chronic unpredictable stress-induced cognitive impairment, neuroinflammation, and biochemical alterations. Naunyn Schmiedeberg’s Arch Pharmacol 387:129–141
Santi A, Baldissareli J, Murussi CR, Dias GR, de Menezes CC, Zanini D, Abdalla FH, Thomé GR, Martins CC, Schetinger MR, Loro VL (2014) Effects of quercetin on oxidative stress biomarkers in methimazole - induced hypothyroid rats. Exp Clin Endocrinol Diabetes 122:533–539
Saravanan G, Ponmurugan P (2012) Antidiabetic effect of S-allylcysteine: effect on thyroid hormone and circulatory antioxidant system in experimental diabetic rats. J Diabetes Complicat 26:280–285
Sherin A, Anu J, Peeyush KT, Smijin S, Anitha M, Roshni BT, Paulose CS (2012) Cholinergic and GABAergic receptor functional deficit in the hippocampus of insulin-induced hypoglycemic and streptozotocin-induced diabetic rats. Neuroscience 202:69–76
Stanley Mainzen Prince P, Kamalakkannan N (2006) Rutin improves glucose homeostasis in streptozotocin diabetic tissues by altering glycolytic and gluconeogenic enzymes. J Biochem Mol Toxicol 20:96–102
Toldy A, Stadler K, Sasvari M, Jakus J, Jung KJ, Chung HY, Berkes I, Nyakas C, Radak Z (2005) The effect of exercise and nettle supplementation on oxidative stress markers in the rat brain. Brain Res Bull 65:487–493
Udayabanu M, Kumaran D, Katyal A (2012) Free chelatable zinc modulates the cholinergic function during hypobaric hypoxia-induced neuronal damage: an in vivo study. Neuroscience 202:434–445
Vessal M, Hemmati M, Vasei M (2003) Antidiabetic effects of quercetin in streptozocin-induced diabetic rats. Comp Biochem Physiol C Toxicol Pharmacol 135C:357–364
Volpicelli-Daley LA, Hrabovska A, Duysen EG, Ferguson SM, Blakely RD, Lockridge O, Levey AI (2003) Altered striatal function and muscarinic cholinergic receptors in acetylcholinesterase knockout mice. Mol Pharmacol 64:1309–1316
Wang SB, Jia JP (2014) Oxymatrine attenuates diabetes-associated cognitive deficits in rats. Acta Pharmacol Sin 35:331–338
Wolff SP, Dean RT (1987) Glucose autoxidation and protein modification. The potential role of ‘autoxidative glycosylation’ in diabetes. Biochem J 245:243–250
Yuede CM, Dong H, Csernansky JG (2007) Anti-dementia drugs and hippocampal-dependent memory in rodents. Behav Pharmacol 18:347–363
Zhang WY, Lee JJ, Kim Y, Kim IS, Park JS, Myung CS (2010) Amelioration of insulin resistance by scopoletin in high-glucose-induced, insulin-resistant HepG2 cells. Horm Metab Res 42:930–935
Acknowledgments
Authors would like to thank Council of Scientific & Industrial Research (India) for financial assistance as Senior Research Fellowship (09/957/0002/2012/EMR-I).
Conflict of interest
The authors declare that they have no conflict of interests.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Suppl. 1
Effect of Urtica dioica extract on streptozotocin-induced alteration in fasting blood glucose level. Data were mean ± SEM values. Significant difference: *CTRL vs STZ, αSTZ vs UD50, βSTZ vs ROSI. *p < 0.05, ***p < 0.001. Reprinted from (Patel and Udayabanu 2013), Copyright © 2014, with permission from Elsevier. (PPTX 71 kb)
Suppl. 2
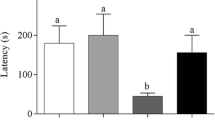
Effect of hydroalcoholic extract of Urtica dioica on streptozotocin induced memory dysfunction in Morris water maze learning trial (a), probe trial (b) and Passive avoidance step through task (c). Data were mean ± SEM values. Significant differences: *CTRL vs STZ, αSTZ vs UD50, βSTZ vs ROSI. *p < 0.05, **p < 0.01, ***p < 0.001. Reprinted from (Patel and Udayabanu 2013), Copyright © 2014, with permission from Elsevier. (PPTX 89 kb)
Suppl. 3
Effect of UD extract on diabetes mediated alterations in the relative OD of (M1) AchR mRNA in striatum (a) and (M4) AchR mRNA expression in hippocampus (b). Data were mean ± SEM values (n = 5). (PPTX 93 kb)
Rights and permissions
About this article
Cite this article
Patel, S.S., Parashar, A. & Udayabanu, M. Urtica dioica leaves modulates muscarinic cholinergic system in the hippocampus of streptozotocin-induced diabetic mice. Metab Brain Dis 30, 803–811 (2015). https://doi.org/10.1007/s11011-014-9646-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11011-014-9646-9