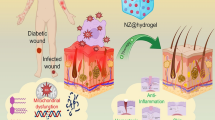
Abstract
Chronic wounds with high disability are among the most common and serious complications of diabetes. Angiogenesis dysfunction impair wound healing in patients with diabetes. Compared with traditional therapies that can only provide symptomatic treatment, stem cells—owing to their powerful paracrine properties, can alleviate the pathogenesis of chronic diabetic wounds and even cure them. Exosome-derived microRNAs (miRNAs), important components of stem cell paracrine signaling, have been reported for therapeutic use in various disease models, including diabetic wounds. Exosome-derived miRNAs have been widely reported to be involved in regulating vascular function and have promising applications in the repair and regeneration of skin wounds. Therefore, this article aims to review the current status of the pathophysiology of exosome-derived miRNAs in the diabetes-induced impairment of wound healing, along with current knowledge of the underlying mechanisms, emphasizing the regulatory mechanism of angiogenesis, we hope to document the emerging theoretical basis for improving wound repair by restoring angiogenesis in diabetes.



Similar content being viewed by others
Data availability
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
Abbreviations
- ADSC:
-
Adipose-derived stem cell
- AGE:
-
Advanced glycation end product
- AGER:
-
Advanced glycosylation end product-specific receptor
- AGO:
-
Argonaute
- CARD10:
-
Caspase recruitment domain protein-10
- circRNAs:
-
Circular RNAs
- CSE:
-
Cystathionine γ-lyase
- DFU:
-
Diabetic foot ulcer
- DM:
-
Diabetes mellitus
- DNMT:
-
DNA methyltransferase
- EC:
-
Endothelial cell
- ECM:
-
Extracellular matrix
- eNOS:
-
Endothelial including nitric oxide synthase
- EPCs:
-
Endothelial progenitor cells
- ER:
-
Endoplasmic reticulum
- GAPDH:
-
Glyceraldehyde 3-phosphate dehydrogenase
- GATA2:
-
Globin transcription factor-binding protein 2
- HbA1c:
-
Glycated hemoglobin A1c
- HG:
-
High glucose
- HUVEC:
-
Human umbilical vein endothelial cell
- HIF:
-
Hypoxia-induced factor
- IL:
-
Interleukin
- lncRNA:
-
Long ncRNAs
- miRNA:
-
MicroRNA
- MSCs:
-
Mesenchymal stem cells
- MMP:
-
Matrix metalloproteinase
- mRNAs:
-
Messenger RNAs
- NADPH:
-
Nicotinamide adenine dinucleotide phosphate
- ncRNA:
-
Non-coding RNA
- NF-κB:
-
Nuclear factor kappa-B
- NO:
-
Nitric oxide
- piRNAs:
-
Piwi RNAs
- pri-miRNA:
-
Primary miRNA transcript
- ROS:
-
Reactive oxygen species
- RAGE:
-
Receptor for advanced glycation end product
- RISC:
-
RNA-induced silencing complex
- ROCK1:
-
Rho-associated coiled-coil kinase 1
- siRNA:
-
Small interfering RNA
- SIRT1:
-
Silent information regulator 1
- SPRED1:
-
Sprouty-related EVH1 domain-containing protein1
- TGF-β:
-
Transforming growth factor-beta
- TNF-α:
-
Tumor necrosis factor-alpha
- UTRs:
-
Untranslated regions
- VEGF:
-
Vascular endothelial growth factor
- VEGFA:
-
Vascular endothelial growth factor A
- VEGFR:
-
Vascular endothelial growth factor receptor
- VCAM-1:
-
Vascular endothelial adhesion molecule 1
References
Sorg H, Tilkorn DJ, Hager S, Hauser J, Mirastschijski U (2017) Skin wound healing: an update on the current knowledge and concepts. Eur Surg Res 58(1–2):81–94
Badr G (2013) Camel whey protein enhances diabetic wound healing in a streptozotocin-induced diabetic mouse model: the critical role of β-defensin-1, -2 and -3. Lipids Health Dis 12:46
Bevan D, Gherardi E, Fan TP, Edwards D, Warn R (2004) Diverse and potent activities of HGF/SF in skin wound repair. J Pathol 203(3):831–838
Sun H, Saeedi P, Karuranga S et al (2022) IDF diabetes atlas: global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract 183:109119
Rao MR, Shen XH, Zou X (1987) Calcium antagonistic action of saponins from Panax notoginseng (sanqi-ginseng). J Tradit Chin Med 7(2):127–130
Wang Y, Chen X, Cao W et al (2014) Plasticity of mesenchymal stem cells in immunomodulation: pathological and therapeutic implications. Nat Immunol 15(11):1009–1016. https://doi.org/10.1038/ni.3002
Smalheiser NR (2007) Exosomal transfer of proteins and RNAs at synapses in the nervous system. Biol Direct 2:35
Hade MD, Suire CN, Suo Z (2021) Mesenchymal stem cell-derived exosomes: applications in regenerative medicine. Cells 10(8):1959
Ke X, Yang R, Wu F et al (2021) Exosomal miR-218-5p/miR-363-3p from endothelial progenitor cells ameliorate myocardial infarction by targeting the p53/JMY signaling pathway. Oxid Med Cell Longev 2021:5529430
Lou R, Chen J, Zhou F, Wang C, Leung CH, Lin L (2022) Exosome-cargoed microRNAs: potential therapeutic molecules for diabetic wound healing. Drug Discov Today 27:103323. https://doi.org/10.1016/j.drudis.2022.07.008
Meng Z, Zhou D, Gao Y et al (2018) miRNA delivery for skin wound healing. Adv Drug Deliv Rev 129:308–318. https://doi.org/10.1016/j.addr.2017.12.011
Cooke JP (2019) Inflammation and its role in regeneration and repair. Circ Res 124(8):1166–1168
Singer AJ, Clark RA (1999) Cutaneous wound healing[J]. N Engl J Med 341(10):738–746. https://doi.org/10.1056/NEJM199909023411006
Griffin DR, Archang MM, Kuan CH et al (2021) Activating an adaptive immune response from a hydrogel scaffold imparts regenerative wound healing. Nat Mater 20(4):560–569. https://doi.org/10.1038/s41563-020-00844-w
Zhang Y, Sun X, Icli B et al (2017) Emerging roles for microRNAs in diabetic microvascular disease: novel targets for therapy. Endocr Rev 38(2):145–168. https://doi.org/10.1210/er.2016-1122
Shen YI, Cho H, Papa AE et al (2016) Engineered human vascularized constructs accelerate diabetic wound healing. Biomaterials 102:107–119. https://doi.org/10.1016/j.biomaterials.2016.06.009
Woo K, Ayello EA, Sibbald RG (2007) The edge effect: current therapeutic options to advance the wound edge. Adv Skin Wound Care 20(2):99–117
Lyttle BD, Vaughn AE, Bardill JR et al (2023) Effects of microRNAs on angiogenesis in diabetic wounds. Front Med 10:1140979. https://doi.org/10.3389/fmed.2023.1140979
Torre-Blanco A, Adachi E, Hojima Y, Wootton JA, Minor RR, Prockop DJ (1992) Temperature-induced post-translational over-modification of type I procollagen. Effects of over-modification of the protein on the rate of cleavage by procollagen N-proteinase and on self-assembly of collagen into fibrils. J Biol Chem 267(4):2650–2655
Yang CL, Rui H, Mosler S, Notbohm H, Sawaryn A, Müller PK (1993) Collagen II from articular cartilage and annulus fibrosus. Structural and functional implication of tissue specific posttranslational modifications of collagen molecules. Eur J Biochem 213(3):1297–1302
Canty EG, Lu Y, Meadows RS, Shaw MK, Holmes DF, Kadler KE (2004) Coalignment of plasma membrane channels and protrusions (fibripositors) specifies the parallelism of tendon. J Cell Biol 165(4):553–563
Khalid M, Petroianu G, Adem A (2022) Advanced glycation end products and diabetes mellitus: mechanisms and perspectives. Biomolecules. https://doi.org/10.3390/biom12040542
Peppa M, Stavroulakis P, Raptis SA (2009) Advanced glycoxidation products and impaired diabetic wound healing. Wound Repair Regen 17(4):461–472
Hu H, Han CM, Hu XL, Ye WL, Huang WJ, Smit AJ (2012) Elevated skin autofluorescence is strongly associated with foot ulcers in patients with diabetes: a cross-sectional, observational study of Chinese subjects. J Zhejiang Univ Sci B 13(5):372–377
Feng X, Tonnesen MG, Mousa SA, Clark RA (2013) Fibrin and collagen differentially but synergistically regulate sprout angiogenesis of human dermal microvascular endothelial cells in 3-dimensional matrix. Int J Cell Biol 2013:231279
Yamauchi M, Sricholpech M (2012) Lysine post-translational modifications of collagen. Essays Biochem 52:113–133
Avery NC, Bailey AJ (2006) The effects of the Maillard reaction on the physical properties and cell interactions of collagen. Pathol Biol (Paris) 54(7):387–395
Verzijl N, DeGroot J, Thorpe SR et al (2000) Effect of collagen turnover on the accumulation of advanced glycation end products. J Biol Chem 275(50):39027–39031
Liao H, Zakhaleva J, Chen W (2009) Cells and tissue interactions with glycated collagen and their relevance to delayed diabetic wound healing. Biomaterials 30(9):1689–1696
Niu Y, Xie T, Ge K, Lin Y, Lu S (2008) Effects of extracellular matrix glycosylation on proliferation and apoptosis of human dermal fibroblasts via the receptor for advanced glycosylated end products. Am J Dermatopathol 30(4):344–351
Khalid M, Alkaabi J, Khan M, Adem A (2021) Insulin signal transduction perturbations in insulin resistance. Int J Mol Sci 22(16):8590
Giacco F, Brownlee M (2010) Oxidative stress and diabetic complications. Circ Res 107(9):1058–1070
Peppa M, Raptis SA (2011) Glycoxidation and wound healing in diabetes: an interesting relationship. Curr Diabetes Rev 7(6):416–425
Vlassara H, Striker GE (2011) AGE restriction in diabetes mellitus: a paradigm shift. Nat Rev Endocrinol 7(9):526–539
Fukui M, Tanaka M, Senmaru T et al (2013) LOX-1 is a novel marker for peripheral artery disease in patients with type 2 diabetes. Metabolism 62(7):935–938
Leguina-Ruzzi A, Pereira J, Pereira-Flores K et al (2015) Increased RhoA/Rho-Kinase activity and markers of endothelial dysfunction in young adult subjects with metabolic syndrome. Metab Syndr Relat Disord 13(9):373–380
Zhang L, Dong L, Liu X et al (2014) α-Melanocyte-stimulating hormone protects retinal vascular endothelial cells from oxidative stress and apoptosis in a rat model of diabetes. PLoS ONE 9(4):e93433
Wang G, Li W, Chen Q, Jiang Y, Lu X, Zhao X (2015) Hydrogen sulfide accelerates wound healing in diabetic rats. Int J Clin Exp Pathol 8(5):5097–5104
Pavkov ME, Weil EJ, Fufaa GD et al (2016) Tumor necrosis factor receptors 1 and 2 are associated with early glomerular lesions in type 2 diabetes. Kidney Int 89(1):226–234
Portillo JA, Greene JA, Okenka G et al (2014) CD40 promotes the development of early diabetic retinopathy in mice. Diabetologia 57(10):2222–2231
Portillo JA, Schwartz I, Zarini S et al (2014) Proinflammatory responses induced by CD40 in retinal endothelial and Müller cells are inhibited by blocking CD40-Traf 2,3 or CD40-Traf6 signaling. Invest Ophthalmol Vis Sci 55(12):8590–8597
Abu El-Asrar AM, De Hertogh G, Nawaz MI et al (2015) The tumor necrosis factor superfamily members TWEAK, TNFSF15 and fibroblast growth factor-inducible protein 14 are upregulated in proliferative diabetic retinopathy. Ophthalmic Res 53(3):122–130
Du X, Matsumura T, Edelstein D et al (2003) Inhibition of GAPDH activity by poly(ADP-ribose) polymerase activates three major pathways of hyperglycemic damage in endothelial cells. J Clin Invest 112(7):1049–1057
Apte RS, Chen DS, Ferrara N (2019) VEGF in signaling and disease: beyond discovery and development. Cell 176(6):1248–1264
Arrigo A, Aragona E, Bandello F (2022) VEGF-targeting drugs for the treatment of retinal neovascularization in diabetic retinopathy. Ann Med 54(1):1089–1111. https://doi.org/10.1080/07853890.2022.2064541
Rodrigues M, Xin X, Jee K et al (2013) VEGF secreted by hypoxic Müller cells induces MMP-2 expression and activity in endothelial cells to promote retinal neovascularization in proliferative diabetic retinopathy. Diabetes 62(11):3863–3873
Jiang F, Chen Q, Huang L et al (2016) TNFSF15 inhibits blood retinal barrier breakdown induced by diabetes. Int J Mol Sci 17(5):615
Costa R, Negrão R, Valente I et al (2013) Xanthohumol modulates inflammation, oxidative stress, and angiogenesis in type 1 diabetic rat skin wound healing. J Nat Prod 76(11):2047–2053
Li X, Gan K, Song G, Wang C (2015) VEGF gene transfected umbilical cord mesenchymal stem cells transplantation improve the lower limb vascular lesions of diabetic rats. J Diabetes Complications 29(7):872–881
Semenza GL (2003) Angiogenesis in ischemic and neoplastic disorders. Annu Rev Med 54:17–28
Zhong X, Liao Y, Chen L et al (2015) The MicroRNAs in the Pathogenesis of Metabolic Memory. Endocrinology 156(9):3157–3168. https://doi.org/10.1210/en.2015-1063
Brownlee M (2001) Biochemistry and molecular cell biology of diabetic complications. Nature 414(6865):813–820
Dubey R, Prabhakar PK, Gupta J (2022) Epigenetics: key to improve delayed wound healing in type 2 diabetes. Mol Cell Biochem 477:371–383. https://doi.org/10.1007/s11010-021-04285-0
Reddy MA, Zhang E, Natarajan R (2015) Epigenetic mechanisms in diabetic complications and metabolic memory. Diabetologia 58(3):443–455. https://doi.org/10.1007/s00125-014-3462-y
Gonçalves S, Yin K, Ito Y et al (2021) COX2 regulates senescence secretome composition and senescence surveillance through PGE2. Cell Rep 34(11):108860. https://doi.org/10.1016/j.celrep.2021.108860
Li D, Zhang L, He Y et al (2022) Novel histone post-translational modifications in diabetes and complications of diabetes: the underlying mechanisms and implications. Biomed Pharmacother 156:113984. https://doi.org/10.1016/j.biopha.2022.113984
Toma C, Wagner WR, Bowry S, Schwartz A, Villanueva F (2009) Fate of culture-expanded mesenchymal stem cells in the microvasculature: in vivo observations of cell kinetics. Circ Res 104(3):398–402
Ratajczak MZ, Kucia M, Jadczyk T et al (2012) Pivotal role of paracrine effects in stem cell therapies in regenerative medicine: can we translate stem cell-secreted paracrine factors and microvesicles into better therapeutic strategies. Leukemia 26(6):1166–1173
Denzer K, Kleijmeer MJ, Heijnen HF, Stoorvogel W, Geuze HJ (2000) Exosome: from internal vesicle of the multivesicular body to intercellular signaling device. J Cell Sci 113(Pt 19):3365–3374
Keller S, Ridinger J, Rupp AK, Janssen JW, Altevogt P (2011) Body fluid derived exosomes as a novel template for clinical diagnostics. J Transl Med 9:86
Ghafouri-Fard S, Shoorei H, Mohaqiq M et al (2020) Non-coding RNAs regulate angiogenic processes. Vascul Pharmacol 133–134:106778. https://doi.org/10.1016/j.vph.2020.106778
Mattick JS, Makunin IV (2006) Non-coding RNA. Hum Mol Genet 15:R17-29
Sousa-Franco A, Rebelo K, da Rocha ST (2019) LncRNAs regulating stemness in aging. Aging Cell 18(1):e12870
Matsui M, Corey DR (2017) Non-coding RNAs as drug targets. Nat Rev Drug Discov 16(3):167–179
Sampath D, Liu C, Vasan K et al (2012) Histone deacetylases mediate the silencing of miR-15a, miR-16, and miR-29b in chronic lymphocytic leukemia. Blood 119(5):1162–1172
Friedman RC, Farh KK, Burge CB, Bartel DP (2009) Most mammalian mRNAs are conserved targets of microRNAs. Genome Res 19(1):92–105
Li XH, Gong QM, Ling Y et al (2014) Inherent lipid metabolic dysfunction in glycogen storage disease IIIa. Biochem Biophys Res Commun 455(1–2):90–97
Moutinho C, Esteller M (2017) MicroRNAs and Epigenetics[J]. Adv Cancer Res 135:189–220. https://doi.org/10.1016/bs.acr.2017.06.003
Lee Y, Kim M, Han J et al (2004) MicroRNA genes are transcribed by RNA polymerase II. EMBO J 23(20):4051–4060
Denli AM, Tops BB, Plasterk RH, Ketting RF, Hannon GJ (2004) Processing of primary microRNAs by the Microprocessor complex. Nature 432:231–235. https://doi.org/10.1038/nature03049
Bohnsack MT, Czaplinski K, Gorlich D (2004) Exportin 5 is a RanGTP-dependent dsRNA-binding protein that mediates nuclear export of pre-miRNAs. RNA 10(2):185–191
Kim VN (2005) MicroRNA biogenesis: coordinated cropping and dicing. Nat Rev Mol Cell Biol 6(5):376–385
Borchert GM, Lanier W, Davidson BL (2006) RNA polymerase III transcribes human microRNAs. Nat Struct Mol Biol 13(12):1097–1101
Iqbal MA, Arora S, Prakasam G, Calin GA, Syed MA (2019) MicroRNA in lung cancer: role, mechanisms, pathways and therapeutic relevance. Mol Aspects Med 70:3–20
Maeyama M, Matuoka H, Tuchida Y, Hashimoto Y (1970) Metabolism of neutral steroids in the human fetus. II. Metabolism of progesterone and pregnenolone in anencephalic monsters after delivery. Steroids 15(1):167–80
Lino MM, Simões S, Vilaça A et al (2018) Modulation of angiogenic activity by light-activatable miRNA-loaded nanocarriers. ACS Nano 12(6):5207–5220. https://doi.org/10.1021/acsnano.7b07538
Sun Z, Shi K, Yang S et al (2018) Effect of exosomal miRNA on cancer biology and clinical applications. Mol Cancer 17(1):147. https://doi.org/10.1186/s12943-018-0897-7
Kir D, Schnettler E, Modi S et al (2018) Regulation of angiogenesis by microRNAs in cardiovascular diseases. Angiogenesis 21(4):699–710. https://doi.org/10.1007/s10456-018-9632-7
Zampetaki A, Kiechl S, Drozdov I et al (2010) Plasma microRNA profiling reveals loss of endothelial miR-126 and other microRNAs in type 2 diabetes. Circ Res 107:810–817. https://doi.org/10.1161/CIRCRESAHA.110.226357
Dangwal S, Stratmann B, Bang C et al (2015) Impairment of wound healing in patients With Type 2 diabetes mellitus influences circulating microRNA patterns via inflammatory cytokines. Arterioscler Thromb Vasc Biol 35(6):1480–1488
Sun X, Lin J, Zhang Y et al (2016) MicroRNA-181b improves glucose homeostasis and insulin sensitivity by regulating endothelial function in white adipose tissue. Circ Res 118:810–821. https://doi.org/10.1161/CIRCRESAHA.115.308166
Xiong Y, Chen L, Yu T et al (2020) Inhibition of circulating exosomal microRNA-15a-3p accelerates diabetic wound repair. Aging 12(10):8968–8986
Xiao S, Zhang D, Liu Z et al (2020) Diabetes-induced glucolipotoxicity impairs wound healing ability of adipose-derived stem cells-through the miR-1248/CITED2/HIF-1α pathway. Aging 12(8):6947–6965
Baldeón RL, Weigelt K, de Wit H et al (2014) Decreased serum level of miR-146a as sign of chronic inflammation in type 2 diabetic patients. PLoS ONE 9:e115209. https://doi.org/10.1371/journal.pone.0115209
Feng B, Chen S, McArthur K et al (2011) miR-146a-Mediated extracellular matrix protein production in chronic diabetes complications. Diabetes 60:2975–2984. https://doi.org/10.2337/db11-0478
Liu J, Wang J, Fu W et al (2021) MiR-195–5p and miR-205–5p in extracellular vesicles isolated from diabetic foot ulcer wound fluid decrease angiogenesis by inhibiting VEGFA expression. Aging 13:19805–19821. https://doi.org/10.18632/aging.203393
Tang W, Guo J, Gu R et al (2020) MicroRNA-29b-3p inhibits cell proliferation and angiogenesis by targeting VEGFA and PDGFB in retinal microvascular endothelial cells. Mol Vis 26:64–75
Wang H, Wang X, Liu X et al (2022) miR-199a-5p plays a pivotal role on wound healing via suppressing VEGFA and ROCK1 in diabetic ulcer foot. Oxid Med Cell Longev 2022:4791059. https://doi.org/10.1155/2022/4791059
Lin CJ, Lan YM, Ou MQ, Ji LQ, Lin SD (2019) Expression of miR-217 and HIF-1α/VEGF pathway in patients with diabetic foot ulcer and its effect on angiogenesis of diabetic foot ulcer rats. J Endocrinol Invest 42:1307–1317. https://doi.org/10.1007/s40618-019-01053-2
Kujawa M, O’Meara M, Li H et al (2022) MicroRNA-466 and microRNA-200 increase endothelial permeability in hyperglycemia by targeting Claudin-5. Mol Ther Nucleic Acids 29:259–271. https://doi.org/10.1016/j.omtn.2022.07.002
Chan YC, Roy S, Khanna S, Sen CK (2012) Downregulation of endothelial microRNA-200b supports cutaneous wound angiogenesis by desilencing GATA binding protein 2 and vascular endothelial growth factor receptor 2. Arterioscler Thromb Vasc Biol 32:1372–1382. https://doi.org/10.1161/ATVBAHA.112.248583
Xiao X, Xu M, Yu H et al (2021) Mesenchymal stem cell-derived small extracellular vesicles mitigate oxidative stress-induced senescence in endothelial cells via regulation of miR-146a/Src. Signal Transduct Target Ther 6:354. https://doi.org/10.1038/s41392-021-00765-3
Mortuza R, Feng B, Chakrabarti S (2014) miR-195 regulates SIRT1-mediated changes in diabetic retinopathy. Diabetologia 57:1037–1046. https://doi.org/10.1007/s00125-014-3197-9
Zheng D, Ma J, Yu Y et al (2015) Silencing of miR-195 reduces diabetic cardiomyopathy in C57BL/6 mice. Diabetologia 58:1949–1958. https://doi.org/10.1007/s00125-015-3622-8
Menghini R, Casagrande V, Cardellini M et al (2009) MicroRNA 217 modulates endothelial cell senescence via silent information regulator 1. Circulation 120:1524–1532. https://doi.org/10.1161/CIRCULATIONAHA.109.864629
Mocharla P, Briand S, Giannotti G et al (2013) AngiomiR-126 expression and secretion from circulating CD34(+) and CD14(+) PBMCs: role for proangiogenic effects and alterations in type 2 diabetics. Blood 121:226–236. https://doi.org/10.1182/blood-2012-01-407106
Witkowski M, Weithauser A, Tabaraie T et al (2016) Micro-RNA-126 reduces the blood thrombogenicity in diabetes mellitus via targeting of tissue factor. Arterioscler Thromb Vasc Biol 36:1263–1271. https://doi.org/10.1161/ATVBAHA.115.306094
Zhang D, Li Z, Wang Z, Zeng F, Xiao W, Yu A (2019) MicroRNA-126: a promising biomarker for angiogenesis of diabetic wounds treated with negative pressure wound therapy. Diabetes Metab Syndr Obes 12:1685–1696. https://doi.org/10.2147/DMSO.S199705
Harris TA, Yamakuchi M, Ferlito M, Mendell JT, Lowenstein CJ (2008) MicroRNA-126 regulates endothelial expression of vascular cell adhesion molecule 1. Proc Natl Acad Sci U S A 105:1516–1521. https://doi.org/10.1073/pnas.0707493105
Xue WL, Chen RQ, Zhang QQ et al (2020) Hydrogen sulfide rescues high glucose-induced migration dysfunction in HUVECs by upregulating miR-126–3p. Am J Physiol Cell Physiol 318:C857-857C869. https://doi.org/10.1152/ajpcell.00406.2019
Wang HJ, Huang YL, Shih YY, Wu HY, Peng CT, Lo WY (2014) MicroRNA-146a decreases high glucose/thrombin-induced endothelial inflammation by inhibiting NAPDH oxidase 4 expression. Mediators Inflamm 2014:379537. https://doi.org/10.1155/2014/379537
Fulzele S, El-Sherbini A, Ahmad S et al (2015) MicroRNA-146b-3p regulates retinal inflammation by suppressing adenosine deaminase-2 in diabetes. Biomed Res Int 2015:846501. https://doi.org/10.1155/2015/846501
Cowan C, Muraleedharan CK, O’Donnell JJ 3rd et al (2014) MicroRNA-146 inhibits thrombin-induced NF-κB activation and subsequent inflammatory responses in human retinal endothelial cells[J]. Invest Ophthalmol Vis Sci 55(8):4944–4951. https://doi.org/10.1167/iovs.13-13631
Poe AJ, Shah R, Khare D et al (2022) Regulatory role of miR-146a in corneal epithelial wound healing via its inflammatory targets in human diabetic cornea. Ocul Surf 25:92–100. https://doi.org/10.1016/j.jtos.2022.06.001
Yu HY, Meng LF, Lu XH, Liu LH, Ci X, Zhuo Z (2020) Protective effect of miR-146 against kidney injury in diabetic nephropathy rats through mediating the NF-κB signaling pathway. Eur Rev Med Pharmacol Sci 24:3215–3222. https://doi.org/10.26355/eurrev_202003_20688
Li B, Zhou Y, Chen J et al (2021) Long noncoding RNA H19 acts as a miR-29b sponge to promote wound healing in diabetic foot ulcer. FASEB J 35(1):e20526
Xue W, Zhang Q, Chen Y, Zhu Y (2022) Hydrogen sulfide improves angiogenesis by regulating the transcription of pri-miR-126 in diabetic endothelial cells. Cells. https://doi.org/10.3390/cells11172651
Tao SC, Guo SC, Li M et al (2017) Chitosan wound dressings incorporating exosomes derived from microRNA-126-overexpressing synovium mesenchymal stem cells provide sustained release of exosomes and heal full-thickness skin defects in a diabetic rat model. Stem Cells Transl Med 6(3):736–747
Dewberry LC, Niemiec SM, Hilton SA et al (2022) Cerium oxide nanoparticle conjugation to microRNA-146a mechanism of correction for impaired diabetic wound healing. Nanomedicine 40:102483. https://doi.org/10.1016/j.nano.2021.102483
Sener G, Hilton SA, Osmond MJ et al (2020) Injectable, self-healable zwitterionic cryogels with sustained microRNA - cerium oxide nanoparticle release promote accelerated wound healing. Acta Biomater 101:262–272. https://doi.org/10.1016/j.actbio.2019.11.014
Yang M, Li R, Wang X, Liu X, Zhang B, Wang Y (2021) Preparation, characterization and wound healing effect of alginate/chitosan microcapsules loaded with polysaccharides from nostoc commune vaucher. Biomed Mater 16:025015. https://doi.org/10.1088/1748-605X/abd051
Li Q, Zhao H, Chen W, Huang P, Bi J (2019) Human keratinocyte-derived microvesicle miRNA-21 promotes skin wound healing in diabetic rats through facilitating fibroblast function and angiogenesis. Int J Biochem Cell Biol 114:105570. https://doi.org/10.1016/j.biocel.2019.105570
Wu D, Kang L, Tian J et al (2020) Exosomes derived from bone mesenchymal stem cells with the stimulation of Fe(3)O(4) nanoparticles and static magnetic field enhance wound healing through upregulated miR-21-5p. Int J Nanomedicine 15:7979–7993. https://doi.org/10.2147/IJN.S275650
Wei P, Zhong C, Yang X et al (2020) Exosomes derived from human amniotic epithelial cells accelerate diabetic wound healing via PI3K-AKT-mTOR-mediated promotion in angiogenesis and fibroblast function. Burns Trauma 8:tkaa020. https://doi.org/10.1093/burnst/tkaa020
Huang C, Luo W, Wang Q et al (2021) Human mesenchymal stem cells promote ischemic repairment and angiogenesis of diabetic foot through exosome miRNA-21-5p. Stem Cell Res 52:102235
Fernandes H, Zonnari A, Abreu R et al (2022) Extracellular vesicles enriched with an endothelial cell pro-survival microRNA affects skin tissue regeneration. Mol Ther Nucleic Acids 28:307–327. https://doi.org/10.1016/j.omtn.2022.03.018
Yu M, Liu W, Li J et al (2020) Exosomes derived from atorvastatin-pretreated MSC accelerate diabetic wound repair by enhancing angiogenesis via AKT/eNOS pathway. Stem Cell Res Ther 11(1):350
Zhong H, Qian J, Xiao Z et al (2021) MicroRNA-133b inhibition restores EGFR expression and accelerates diabetes-impaired wound healing. Oxid Med Cell Longev 2021:9306760. https://doi.org/10.1155/2021/9306760
Wang JM, Tao J, Chen DD et al (2014) MicroRNA miR-27b rescues bone marrow-derived angiogenic cell function and accelerates wound healing in type 2 diabetes mellitus. Arterioscler Thromb Vasc Biol 34:99–109. https://doi.org/10.1161/ATVBAHA.113.302104
Sakshi S, Jayasuriya R, Sathish Kumar RC et al (2023) MicroRNA-27b impairs Nrf2-mediated angiogenesis in the progression of diabetic foot ulcer. J Clin Med. https://doi.org/10.3390/jcm12134551
Lu Y, Wen H, Huang J et al (2020) Extracellular vesicle-enclosed miR-486-5p mediates wound healing with adipose-derived stem cells by promoting angiogenesis. J Cell Mol Med 24:9590–9604. https://doi.org/10.1111/jcmm.15387
Cai HA, Huang L, Zheng LJ et al (2019) Ginsenoside (Rg-1) promoted the wound closure of diabetic foot ulcer through iNOS elevation via miR-23a/IRF-1 axis. Life Sci 233:116525. https://doi.org/10.1016/j.lfs.2019.05.081
Zhang J, Cai W, Fan Z et al (2019) MicroRNA-24 inhibits the oxidative stress induced by vascular injury by activating the Nrf2/Ho-1 signaling pathway. Atherosclerosis 290:9–18. https://doi.org/10.1016/j.atherosclerosis.2019.08.023
Cai W, Zhang J, Yang J et al (2019) MicroRNA-24 attenuates vascular remodeling in diabetic rats through PI3K/Akt signaling pathway. Nutr Metab Cardiovasc Dis 29:621–632. https://doi.org/10.1016/j.numecd.2019.03.002
Yan C, Chen J, Wang C et al (2022) Milk exosomes-mediated miR-31-5p delivery accelerates diabetic wound healing through promoting angiogenesis. Drug Deliv 29:214–228. https://doi.org/10.1080/10717544.2021.2023699
Cheng HS, Pérez-Cremades D, Zhuang R et al (2023) Impaired angiogenesis in diabetic critical limb ischemia is mediated by a miR-130b/INHBA signaling axis. JCI Insight. https://doi.org/10.1172/jci.insight.163041
Ge L, Wang K, Lin H et al (2023) Engineered exosomes derived from miR-132-overexpresssing adipose stem cells promoted diabetic wound healing and skin reconstruction. Front Bioeng Biotechnol 11:1129538. https://doi.org/10.3389/fbioe.2023.1129538
McCoy MG, Jamaiyar A, Sausen G et al (2023) MicroRNA-375 repression of Kruppel-like factor 5 improves angiogenesis in diabetic critical limb ischemia. Angiogenesis 26:107–127. https://doi.org/10.1007/s10456-022-09856-3
Shi R, Jin Y, Zhao S, Yuan H, Shi J, Zhao H (2022) Hypoxic ADSC-derived exosomes enhance wound healing in diabetic mice via delivery of circ-Snhg11 and induction of M2-like macrophage polarization. Biomed Pharmacother 153:113463. https://doi.org/10.1016/j.biopha.2022.113463
Yu M, Huang J, Zhu T et al (2020) Liraglutide-loaded PLGA/gelatin electrospun nanofibrous mats promote angiogenesis to accelerate diabetic wound healing via the modulation of miR-29b-3p. Biomater Sci 8:4225–4238. https://doi.org/10.1039/d0bm00442a
Xu Y, Yu T, He L et al (2020) Inhibition of miRNA-152–3p enhances diabetic wound repair via upregulation of PTEN. Aging 12:14978–14989. https://doi.org/10.18632/aging.103557
Han ZF, Cao JH, Liu ZY, Yang Z, Qi RX, Xu HL (2022) Exosomal lncRNA KLF3-AS1 derived from bone marrow mesenchymal stem cells stimulates angiogenesis to promote diabetic cutaneous wound healing. Diabetes Res Clin Pract 183:109126. https://doi.org/10.1016/j.diabres.2021.109126
Pizzino G, Irrera N, Galfo F et al (2018) Effects of the antagomiRs 15b and 200b on the altered healing pattern of diabetic mice. Br J Pharmacol 175:644–655. https://doi.org/10.1111/bph.14113
Qi L, Lu Y, Wang Z, Zhang G (2021) microRNA-106b derived from endothelial cell-secreted extracellular vesicles prevents skin wound healing by inhibiting JMJD3 and RIPK3. J Cell Mol Med 25:4551–4561. https://doi.org/10.1111/jcmm.16037
Liang ZH, Pan NF, Lin SS et al (2022) Exosomes from mmu_circ_0001052-modified adipose-derived stem cells promote angiogenesis of DFU via miR-106a-5p and FGF4/p38MAPK pathway. Stem Cell Res Ther 13:336. https://doi.org/10.1186/s13287-022-03015-7
Chen X, Yang R, Wang J et al (2020) Porcine acellular dermal matrix accelerates wound healing through miR-124-3p.1 and miR-139-5p. Cytotherapy 22:494–502. https://doi.org/10.1016/j.jcyt.2020.04.042
Liang F, Luo YF, Guo Z, Qian Q, Meng XB, Mo ZH (2023) MicroRNA-139-5p mediates BMSCs impairment in diabetes by targeting HOXA9/c-Fos. FASEB J 37:e22697. https://doi.org/10.1096/fj.202201059R
Shi R, Jin Y, Hu W et al (2020) Exosomes derived from mmu_circ_0000250-modified adipose-derived mesenchymal stem cells promote wound healing in diabetic mice by inducing miR-128–3p/SIRT1-mediated autophagy. Am J Physiol Cell Physiol 318:C848-848C856. https://doi.org/10.1152/ajpcell.00041.20204
Huang L, Cai HA, Zhang MS, Liao RY, Huang X, Hu FD (2021) Ginsenoside Rg1 promoted the wound healing in diabetic foot ulcers via miR-489-3p/Sirt1 axis. J Pharmacol Sci 147:271–283. https://doi.org/10.1016/j.jphs.2021.07.008
Lucas T, Schäfer F, Müller P, Eming SA, Heckel A, Dimmeler S (2017) Light-inducible antimiR-92a as a therapeutic strategy to promote skin repair in healing-impaired diabetic mice. Nat Commun 8:15162. https://doi.org/10.1038/ncomms15162
Li H, Chang L, Du WW et al (2014) Anti-microRNA-378a enhances wound healing process by upregulating integrin beta-3 and vimentin. Mol Ther 22:1839–1850. https://doi.org/10.1038/mt.2014.115
Wang JM, Qiu Y, Yang ZQ, Li L, Zhang K (2017) Inositol-requiring enzyme 1 facilitates diabetic wound healing through modulating MicroRNAs. Diabetes 66:177–192. https://doi.org/10.2337/db16-0052
Icli B, Wu W, Ozdemir D et al (2019) MicroRNA-135a-3p regulates angiogenesis and tissue repair by targeting p38 signaling in endothelial cells. FASEB J 33:5599–5614. https://doi.org/10.1096/fj.201802063RR
Icli B, Nabzdyk CS, Lujan-Hernandez J et al (2016) Regulation of impaired angiogenesis in diabetic dermal wound healing by microRNA-26a. J Mol Cell Cardiol 91:151–159. https://doi.org/10.1016/j.yjmcc.2016.01.007
Asadi-Yousefabad SL, Nammian P, Tabei S et al (2022) Angiogenesis in diabetic mouse model with critical limb ischemia; cell and gene therapy. Microvasc Res 141:104339
Niemiec SM, Louiselle AE, Hilton SA et al (2020) Nanosilk increases the strength of diabetic skin and delivers CNP-miR146a to improve wound healing. Front Immunol 11:590285
Stager MA, Bardill J, Raichart A et al (2022) Photopolymerized zwitterionic hydrogels with a sustained delivery of cerium oxide nanoparticle-miR146a conjugate accelerate diabetic wound healing. ACS Appl Bio Mater 5(3):1092–1103
Wu D, Chang X, Tian J et al (2021) Bone mesenchymal stem cells stimulation by magnetic nanoparticles and a static magnetic field: release of exosomal miR-1260a improves osteogenesis and angiogenesis. J Nanobiotechnology 19(1):209
Wang J, Wu H, Peng Y et al (2021) Hypoxia adipose stem cell-derived exosomes promote high-quality healing of diabetic wound involves activation of PI3K/Akt pathways. J Nanobiotechnology 19(1):202
Galiano RD, Michaels J, Dobryansky M et al (2004) Quantitative and reproducible murine model of excisional wound healing. Wound Repair Regen 12(4):485–92
Acknowledgements
The authors are grateful for financial supports from the National Natural Science Foundation of China, the Ministry of the Science and Technology, the Guizhou Provincial Department of Science and Technology, and the Guizhou Provincial Department of Education, P.R.China.
Funding
National Natural Science Foundation of China (Nos. 81460156, 31960191), the Science and Technology Innovation Leading Academics of National High-level Personnel of Special Support Program (No. GKFZ-2018-29), the High-Level Innovative Talent Support Program of Guizhou Province (No. QKHPT-RC-GCC[2022]001-1), the Natural Science Foundation of Guizhou Province (No. QKHJC-ZK-2021-ZD-026), and the Special Funds from the Central Government to Support the Development of Local Colleges and Universities - the Construction Project of Key Laboratory in Guizhou Province (No. QJJ[2023]020), P.R.China.
Author information
Authors and Affiliations
Contributions
JHX contributed to the study conception and design, supervision, and critical revision. Material preparation, data collection and analysis, and writing of original draft were performed by WTC. YL and XMC revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, WT., Luo, Y., Chen, XM. et al. Role of exosome-derived miRNAs in diabetic wound angiogenesis. Mol Cell Biochem (2023). https://doi.org/10.1007/s11010-023-04874-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11010-023-04874-1