Abstract
Migraine is a debilitating disorder that afflicts over 1 billion people worldwide, involving attacks that result in a throbbing and pulsating headache. Migraine is thought to be a neurovascular event associated with vasoconstriction, vasodilation, and neuronal activation. Understanding signaling in migraine pathology is central to the development of therapeutics for migraine prophylaxis and for mitigation of migraine in the prodrome phase before pain sets in. The fact that both vasoactivity and neural sensitization are involved in migraine indicates that agonists which promote these phenomena may very well be involved in migraine pathology. One such group of agonists is the purines, in particular, adenosine phosphates and their metabolites. This manuscript explores what is known about the relationship between these metabolites and migraine pathology and explores the potential for such relationships through their known signaling pathways.
Graphical abstract

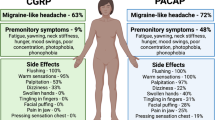
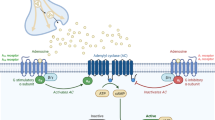
Reported receptor involvement in vasoaction and nociception


Similar content being viewed by others
Data availability
Not applicable.
Code availability
Not applicable.
References
Robbins MS (2021) Diagnosis and management of headache: a review. JAMA 325(18):1874–1885. https://doi.org/10.1001/jama.2021.1640
Ashina M, Katsarava Z, Do TP, Buse DC, Pozo-Rosich P, Özge A, Krymchantowski AV, Lebedeva ER, Ravishankar K, Yu S, Sacco S, Ashina S, Younis S, Steiner TJ, Lipton RB (2021) Migraine: epidemiology and systems of care. Lancet 397(10283):1485–1495. https://doi.org/10.1016/S0140-6736(20)32160-7
Diamond S (1991) Strategies for migraine management. Cleve Clin J Med 58(3):257–261. https://doi.org/10.3949/ccjm.58.3.257
Meyer JS, Terayama Y, Takashima S, Obara K (1993) Cerebral circulatory changes during migraine headache with aura. Rev Neurosci 4(3):305–319. https://doi.org/10.1515/revneuro.1993.4.3.305
Brennan KC, Charles A (2010) An update on the blood vessel in migraine. Curr Opin Neurol 23(3):266–274. https://doi.org/10.1097/WCO.0b013e32833821c1
Burstein R, Noseda R, Borsook D (2015) Migraine: multiple processes, complex pathophysiology. J Neurosci 35(17):6619–6629. https://doi.org/10.1523/JNEUROSCI.0373-15.2015
Jacobs B, Dussor G (2016) Neurovascular contributions to migraine: Moving beyond vasodilation. Neuroscience 338:130–144. https://doi.org/10.1016/j.neuroscience.2016.06.012
Hoffmann J, Baca SM, Akerman S (2019) Neurovascular mechanisms of migraine and cluster headache. J Cereb Blood Flow Metab 39(4):573–594. https://doi.org/10.1177/0271678X17733655
Graham JR, Wolff HG (1938) Mechanism of migraine headache and action of ergotamine tartrate. Arch Neurol Psychiatry 39(4):737–763
Dukes HT, Vieth RG (1964) Cerebral arteriography during migraine prodrome and headache. Neurology 14:636–639. https://doi.org/10.1212/wnl.14.7.636
Skinhoj E, Paulson OB (1969) Regional blood flow in internal carotid distribution during migraine attack. Br Med J 3(5670):569–570. https://doi.org/10.1136/bmj.3.5670.569
Masuzawa T, Shinoda S, Furuse M, Nakahara N, Abe F, Sato F (1983) Cerebral angiographic changes on serial examination of a patient with migraine. Neuroradiology 24(5):277–281. https://doi.org/10.1007/BF00333181
Lieberman AN, Jonas S, Hass WK, Pinto R, Lin J, Leibowitz M, Hassouri H (1984) Bilateral cervical carotid and intracranial vasospasm causing cerebral ischemia in a migrainous patient: a case of “diplegic migraine.” Headache 24(5):245–248. https://doi.org/10.1111/j.1526-4610.1984.hed2405245.x
Moskowitz MA, Reinhard JF Jr, Romero J, Melamed E, Pettibone DJ (1979) Neurotransmitters and the fifth cranial nerve: is there a relation to the headache phase of migraine? Lancet 2(8148):883–885. https://doi.org/10.1016/s0140-6736(79)92692-8
May A, Goadsby PJ (1999) The trigeminovascular system in humans: pathophysiologic implications for primary headache syndromes of the neural influences on the cerebral circulation. J Cereb Blood Flow Metab 19(2):115–127. https://doi.org/10.1097/00004647-199902000-00001
Tepper SJ, Rapoport A, Sheftell F (2001) The pathophysiology of migraine. Neurologist 7(5):279–286. https://doi.org/10.1097/00127893-200109000-00002
Ashina M, Hansen JM, Do TP, Melo-Carrillo A, Burstein R, Moskowitz MA (2019) Migraine and the trigeminovascular system-40 years and counting. Lancet Neurol 18(8):795–804. https://doi.org/10.1016/S1474-4422(19)30185-1
Haanes KA, Labastida-Ramírez A, Blixt FW, Rubio-Beltrán E, Dirven CM, Danser AH, Edvinsson L, MaassenVanDenBrink A (2019) Exploration of purinergic receptors as potential anti-migraine targets using established pre-clinical migraine models. Cephalalgia 39(11):1421–1434. https://doi.org/10.1177/0333102419851810
Burnstock G (1981) Pathophysiology of migraine: a new hypothesis. Lancet 1(8235):1397–1399. https://doi.org/10.1016/s0140-6736(81)92572-1
Burnstock G (2008) Purinergic signalling and disorders of the central nervous system. Nat Rev Drug Discov 7(7):575–590. https://doi.org/10.1038/nrd2605
Burnstock G (2009) Purines and sensory nerves. Handb Exp Pharmacol 194:333–392. https://doi.org/10.1007/978-3-540-79090-7_10
Burnstock G, Ralevic V (2013) Purinergic signaling and blood vessels in health and disease. Pharmacol Rev 66(1):102–192. https://doi.org/10.1124/pr.113.008029
Seminario-Vidal L, Lazarowski ER, Okada SF (2009) Assessment of extracellular ATP concentrations. Methods Mol Biol 574:25–36. https://doi.org/10.1007/978-1-60327-321-3_3
Soslau G (2019) Extracellular adenine compounds within the cardiovascular system: Their source, metabolism and function. Med Drug Discov 4:100018. https://doi.org/10.1016/j.medidd.2020.100018
Zimmermann H (2000) Extracellular metabolism of ATP and other nucleotides. Naunyn Schmiedebergs Arch Pharmacol 362(4–5):299–309. https://doi.org/10.1007/s002100000309
Born GV, Kratzer MA (1984) Source and concentration of extracellular adenosine triphosphate during haemostasis in rats, rabbits and man. J Physiol 354:419–429. https://doi.org/10.1113/jphysiol.1984.sp015385
Hanington E (1989) Migraine: the platelet hypothesis after 10 years. Biomed Pharmacother 43(10):719–726. https://doi.org/10.1016/0753-3322(89)90160-1
Joseph R, Welch KM, D’Andrea G, Levine SR (1986) ATP hyposecretion from platelet dense bodies—evidence for the purinergic hypothesis and a marker of migraine. Headache 26(8):403–410. https://doi.org/10.1111/j.1526-4610.1986.hed2608403.x
Bergfeld GR, Forrester T (1992) Release of ATP from human erythrocytes in response to a brief period of hypoxia and hypercapnia. Cardiovasc Res 26(1):40–47. https://doi.org/10.1093/cvr/26.1.40
Forsyth AM, Wan J, Owrutsky PD, Abkarian M, Stone HA (2011) Multiscale approach to link red blood cell dynamics, shear viscosity, and ATP release. Proc Natl Acad Sci U S A 108(27):10986–10991. https://doi.org/10.1073/pnas.1101315108
Sperlágh B, Vizi SE (1996) Neuronal synthesis, storage and release of ATP. Semin Neurosci 8(4):175–186
Amici A, Grolla AA, Del Grosso E, Bellini R, Bianchi M, Travelli C, Garavaglia S, Sorci L, Raffaelli N, Ruggieri S, Genazzani AA, Orsomando G (2017) Synthesis and degradation of adenosine 5’-tetraphosphate by nicotinamide and nicotinate phosphoribosyltransferases. Cell Chem Biol 24(5):553-564.e4. https://doi.org/10.1016/j.chembiol.2017.03.010
Tölle M, Jankowski V, Schuchardt M, Wiedon A, Huang T, Hub F, Kowalska J, Jemielity J, Guranowski A, Loddenkemper C, Zidek W, Jankowski J, van der Giet M (2008) Adenosine 5’-tetraphosphate is a highly potent purinergic endothelium-derived vasoconstrictor. Circ Res 103(10):1100–1108. https://doi.org/10.1161/CIRCRESAHA.108.177865
Leira R, Castillo J, Martínez F, Castro A, Lema M, Noya M (1991) Nucleótidos de la adenina en la migraña [Adenine nucleotides in migraine]. Neurologia 6(6):207–210 ((in Spanish))
Joseph R, Welch KM, D’Andrea G (1989) Serotonergic hypofunction in migraine: a synthesis of evidence based on platelet dense body dysfunction. Cephalalgia 9(4):293–299. https://doi.org/10.1046/j.1468-2982.1989.0904293.x
Hanington E, Jones RJ, Amess JA (1982) Platelet nucleotides in migraine. Lancet 2(8295):437. https://doi.org/10.1016/s0140-6736(82)90459-7
Hedman C, Winther K, Knudsen JB (1988) Platelet function in classic migraine during attack-free periods. Acta Neurol Scand 78(4):271–277. https://doi.org/10.1111/j.1600-0404.1988.tb03656.x
Kimball RW, Freidman AP (1961) Further studies of neurohumoral agents in patients with vascular headaches. Neurology 11:116–119. https://doi.org/10.1212/wnl.11.2.116
Skrabanja AT, Bouman EA, Dagnelie PC (2005) Potential value of adenosine 5’-triphosphate (ATP) and adenosine in anaesthesia and intensive care medicine. Br J Anaesth 94(5):556–562. https://doi.org/10.1093/bja/aei093
Petrović F, Stojanov D, Aracki-Trenkić A, Petrović J, Petrović M, Janković S (2021) Brain magnetic resonance spectroscopy in migraine. Acta Medica Medianae 60(2):77–87. https://doi.org/10.5633/amm.2021.0210
Barbiroli B, Montagna P, Cortelli P, Funicello R, Iotti S, Monari L, Pierangeli G, Zaniol P, Lugaresi E (1992) Abnormal brain and muscle energy metabolism shown by 31P magnetic resonance spectroscopy in patients affected by migraine with aura. Neurology 42(6):1209–1214. https://doi.org/10.1212/wnl.42.6.1209
Montagna P, Cortelli P, Monari L, Pierangeli G, Parchi P, Lodi R, Iotti S, Frassineti C, Zaniol P, Lugaresi E et al (1994) 31P-magnetic resonance spectroscopy in migraine without aura. Neurology 44(4):666–669. https://doi.org/10.1212/wnl.44.4.666
Lodi R, Montagna P, Soriani S, Iotti S, Arnaldi C, Cortelli P, Pierangeli G, Patuelli A, Zaniol P, Barbiroli B (1997) Deficit of brain and skeletal muscle bioenergetics and low brain magnesium in juvenile migraine: an in vivo 31P magnetic resonance spectroscopy interictal study. Pediatr Res 42(6):866–871. https://doi.org/10.1203/00006450-199712000-00024
Uncini A, Lodi R, Di Muzio A, Silvestri G, Servidei S, Lugaresi A, Iotti S, Zaniol P, Barbiroli B (1995) Abnormal brain and muscle energy metabolism shown by 31P-MRS in familial hemiplegic migraine. J Neurol Sci 129(2):214–222. https://doi.org/10.1016/0022-510x(94)00283-t
Reyngoudt H, Paemeleire K, Descamps B, De Deene Y, Achten E (2011) 31P-MRS demonstrates a reduction in high-energy phosphates in the occipital lobe of migraine without aura patients. Cephalalgia 31(12):1243–1253. https://doi.org/10.1177/0333102410394675
Schulz UG, Blamire AM, Corkill RG, Davies P, Styles P, Rothwell PM (2007) Association between cortical metabolite levels and clinical manifestations of migrainous aura: an MR-spectroscopy study. Brain 130(Pt 12):3102–3110. https://doi.org/10.1093/brain/awm165
Guieu R, Sampiéri F, Bechis G, Rochat H (1994) Use of HPLC to measure circulating adenosine levels in migrainous patients. Clin Chim Acta 227(1–2):185–194. https://doi.org/10.1016/0009-8981(94)90146-5
Guieu R, Devaux C, Henry H, Bechis G, Pouget J, Mallet D, Sampieri F, Juin M, Gola R, Rochat H (1998) Adenosine and migraine. Can J Neurol Sci 25(1):55–58. https://doi.org/10.1017/s0317167100033497
North RA (2002) Molecular physiology of P2X receptors. Physiol Rev 82(4):1013–1067. https://doi.org/10.1152/physrev.00015.2002
Khakh BS (2001) Molecular physiology of P2X receptors and ATP signalling at synapses. Nat Rev Neurosci 2(3):165–174. https://doi.org/10.1038/35058521
Illes P, Müller CE, Jacobson KA, Grutter T, Nicke A, Fountain SJ, Kennedy C, Schmalzing G, Jarvis MF, Stojilkovic SS, King BF, Di Virgilio F (2021) Update of P2X receptor properties and their pharmacology: IUPHAR Review 30. Br J Pharmacol 178(3):489–514. https://doi.org/10.1111/bph.15299
Evans RJ, Lewis C, Buell G, Valera S, North RA, Surprenant A (1995) Pharmacological characterization of heterologously expressed ATP-gated cation channels (P2x purinoceptors). Mol Pharmacol 48(2):178–183
Li M, Silberberg SD, Swartz KJ (2013) Subtype-specific control of P2X receptor channel signaling by ATP and Mg2+. Proc Natl Acad Sci U S A 110(36):E3455–E3463. https://doi.org/10.1073/pnas.1308088110
Valera S, Hussy N, Evans RJ, Adami N, North RA, Surprenant A, Buell G (1994) A new class of ligand-gated ion channel defined by P2x receptor for extracellular ATP. Nature 371(6497):516–519. https://doi.org/10.1038/371516a0
Valera S, Talabot F, Evans RJ, Gos A, Antonarakis SE, Morris MA, Buell GN (1995) Characterization and chromosomal localization of a human P2X receptor from the urinary bladder. Recept Channels 3(4):283–289
Eickhorst AN, Berson A, Cockayne D, Lester HA, Khakh BS (2002) Control of P2X(2) channel permeability by the cytosolic domain. J Gen Physiol 120(2):119–131. https://doi.org/10.1085/jgp.20028535
Liu M, King BF, Dunn PM, Rong W, Townsend-Nicholson A, Burnstock G (2001) Coexpression of P2X(3) and P2X(2) receptor subunits in varying amounts generates heterogeneous populations of P2X receptors that evoke a spectrum of agonist responses comparable to that seen in sensory neurons. J Pharmacol Exp Ther 296(3):1043–1050
Lynch KJ, Touma E, Niforatos W, Kage KL, Burgard EC, van Biesen T, Kowaluk EA, Jarvis MF (1999) Molecular and functional characterization of human P2X(2) receptors. Mol Pharmacol 56(6):1171–1181. https://doi.org/10.1124/mol.56.6.1171
Séguéla P, Haghighi A, Soghomonian JJ, Cooper E (1996) A novel neuronal P2x ATP receptor ion channel with widespread distribution in the brain. J Neurosci 16(2):448–455. https://doi.org/10.1523/JNEUROSCI.16-02-00448.1996
Ase AR, Honson NS, Zaghdane H, Pfeifer TA, Séguéla P (2015) Identification and characterization of a selective allosteric antagonist of human P2X4 receptor channels. Mol Pharmacol 87(4):606–616. https://doi.org/10.1124/mol.114.096222
Bo X, Zhang Y, Nassar M, Burnstock G, Schoepfer R (1995) A P2X purinoceptor cDNA conferring a novel pharmacological profile. FEBS Lett 375(1–2):129–133. https://doi.org/10.1016/0014-5793(95)01203-q
Buell G, Lewis C, Collo G, North RA, Surprenant A (1996) An antagonist-insensitive P2X receptor expressed in epithelia and brain. EMBO J 15(1):55–62
Garcia-Guzman M, Soto F, Gomez-Hernandez JM, Lund PE, Stühmer W (1997) Characterization of recombinant human P2X4 receptor reveals pharmacological differences to the rat homologue. Mol Pharmacol 51(1):109–118. https://doi.org/10.1124/mol.51.1.109
Bo X, Jiang LH, Wilson HL, Kim M, Burnstock G, Surprenant A, North RA (2003) Pharmacological and biophysical properties of the human P2X5 receptor. Mol Pharmacol 63(6):1407–1416. https://doi.org/10.1124/mol.63.6.1407
Donnelly-Roberts DL, Namovic MT, Han P, Jarvis MF (2009) Mammalian P2X7 receptor pharmacology: comparison of recombinant mouse, rat and human P2X7 receptors. Br J Pharmacol 157(7):1203–1214. https://doi.org/10.1111/j.1476-5381.2009.00233.x
McCarthy AE, Yoshioka C, Mansoor SE (2019) Full-length P2X7 structures reveal how palmitoylation prevents channel desensitization. Cell 179(3):659-670.e13. https://doi.org/10.1016/j.cell.2019.09.017
Rassendren F, Buell GN, Virginio C, Collo G, North RA, Surprenant A (1997) The permeabilizing ATP receptor, P2X7. Cloning and expression of a human cDNA. J Biol Chem 272(9):5482–5486. https://doi.org/10.1074/jbc.272.9.5482
Soares-Bezerra RJ, Ferreira NC, Alberto AV, Bonavita AG, Fidalgo-Neto AA, Calheiros AS, FrutuosoVda S (2015) Alves LA (2005) An improved method for P2X7R antagonist screening. PLoS ONE 10(5):e0123089. https://doi.org/10.1371/journal.pone.0123089
Surprenant A, Rassendren F, Kawashima E, North RA, Buell G (1996) The cytolytic P2Z receptor for extracellular ATP identified as a P2X receptor (P2X7). Science 272(5262):735–738. https://doi.org/10.1126/science.272.5262.735
Nicke A, Bäumert HG, Rettinger J, Eichele A, Lambrecht G, Mutschler E, Schmalzing G (1998) P2X1 and P2X3 receptors form stable trimers: a novel structural motif of ligand-gated ion channels. EMBO J 17(11):3016–3028. https://doi.org/10.1093/emboj/17.11.3016
Becker D, Woltersdorf R, Boldt W, Schmitz S, Braam U, Schmalzing G, Markwardt F (2008) The P2X7 carboxyl tail is a regulatory module of P2X7 receptor channel activity. J Biol Chem 283(37):25725–25734. https://doi.org/10.1074/jbc.M803855200
Aschrafi A, Sadtler S, Niculescu C, Rettinger J, Schmalzing G (2004) Trimeric architecture of homomeric P2X2 and heteromeric P2X1+2 receptor subtypes. J Mol Biol 342(1):333–343. https://doi.org/10.1016/j.jmb.2004.06.092
Barrera NP, Ormond SJ, Henderson RM, Murrell-Lagnado RD, Edwardson JM (2005) Atomic force microscopy imaging demonstrates that P2X2 receptors are trimers but that P2X6 receptor subunits do not oligomerize. J Biol Chem 280(11):10759–10765. https://doi.org/10.1074/jbc.M412265200
Torres GE, Egan TM, Voigt MM (1999) Hetero-oligomeric assembly of P2X receptor subunits. Specificities exist with regard to possible partners. J Biol Chem 274(10):6653–6659. https://doi.org/10.1074/jbc.274.10.6653
Antonio LS, Stewart AP, Xu XJ, Varanda WA, Murrell-Lagnado RD, Edwardson JM (2011) P2X4 receptors interact with both P2X2 and P2X7 receptors in the form of homotrimers. Br J Pharmacol 163(5):1069–1077. https://doi.org/10.1111/j.1476-5381.2011.01303.x
Collo G, North RA, Kawashima E, Merlo-Pich E, Neidhart S, Surprenant A, Buell G (1996) Cloning of P2X5 and P2X6 receptors and the distribution and properties of an extended family of ATP-gated ion channels. J Neurosci 16(8):2495–2507. https://doi.org/10.1523/JNEUROSCI.16-08-02495.1996
Jones CA, Vial C, Sellers LA, Humphrey PP, Evans RJ, Chessell IP (2004) Functional regulation of P2X6 receptors by N-linked glycosylation: identification of a novel alpha beta-methylene ATP-sensitive phenotype. Mol Pharmacol 65(4):979–985. https://doi.org/10.1124/mol.65.4.979
Nicke A, Kerschensteiner D, Soto F (2005) Biochemical and functional evidence for heteromeric assembly of P2X1 and P2X4 subunits. J Neurochem 92(4):925–933. https://doi.org/10.1111/j.1471-4159.2004.02939.x
Harhun MI, Povstyan OV, Albert AP, Nichols CM (2014) ATP-evoked sustained vasoconstrictions mediated by heteromeric P2X1/4 receptors in cerebral arteries. Stroke 45(8):2444–2450. https://doi.org/10.1161/STROKEAHA.114.005544
Lalo U, Pankratov Y, Wichert SP, Rossner MJ, North RA, Kirchhoff F, Verkhratsky A (2008) P2X1 and P2X5 subunits form the functional P2X receptor in mouse cortical astrocytes. J Neurosci 28(21):5473–5480. https://doi.org/10.1523/JNEUROSCI.1149-08.2008
Lê KT, Boué-Grabot E, Archambault V, Séguéla P (1999) Functional and biochemical evidence for heteromeric ATP-gated channels composed of P2X1 and P2X5 subunits. J Biol Chem 274(22):15415–15419. https://doi.org/10.1074/jbc.274.22.15415
Saul A, Hausmann R, Kless A, Nicke A (2013) Heteromeric assembly of P2X subunits. Front Cell Neurosci 7:250. https://doi.org/10.3389/fncel.2013.00250
Compan V, Ulmann L, Stelmashenko O, Chemin J, Chaumont S, Rassendren F (2012) P2X2 and P2X5 subunits define a new heteromeric receptor with P2X7-like properties. J Neurosci 32(12):4284–4296. https://doi.org/10.1523/JNEUROSCI.6332-11.2012
Hausmann R, Bodnar M, Woltersdorf R, Wang H, Fuchs M, Messemer N, Qin Y, Günther J, Riedel T, Grohmann M, Nieber K, Schmalzing G, Rubini P, Illes P (2012) ATP binding site mutagenesis reveals different subunit stoichiometry of functional P2X2/3 and P2X2/6 receptors. J Biol Chem 287(17):13930–13943. https://doi.org/10.1074/jbc.M112.345207
Lê KT, Babinski K, Séguéla P (1998) Central P2X4 and P2X6 channel subunits coassemble into a novel heteromeric ATP receptor. J Neurosci 18(18):7152–7159. https://doi.org/10.1523/JNEUROSCI.18-18-07152.1998
Uhlén M, Fagerberg L, Hallström BM, Lindskog C, Oksvold P, Mardinoglu A, Sivertsson Å, Kampf C, Sjöstedt E, Asplund A, Olsson I, Edlund K, Lundberg E, Navani S, Szigyarto CA, Odeberg J, Djureinovic D, Takanen JO, Hober S, Alm T, Edqvist PH, Berling H, Tegel H, Mulder J, Rockberg J, Nilsson P, Schwenk JM, Hamsten M, von Feilitzen K, Forsberg M, Persson L, Johansson F, Zwahlen M, von Heijne G, Nielsen J, Pontén F (2015) Tissue-based map of the human proteome. Science 347(6220):1260419. https://doi.org/10.1126/science.1260419
Ralevic V, Dunn WR (2015) Purinergic transmission in blood vessels. Auton Neurosci 191:48–66. https://doi.org/10.1016/j.autneu.2015.04.007
Nichols CM, Povstyan OV, Albert AP, Gordienko DV, Khan O, Vasilikostas G, Khong TK, Wan A, Reddy M, Harhun MI (2014) Vascular smooth muscle cells from small human omental arteries express P2X1 and P2X4 receptor subunits. Purinergic Signal 10(4):565–572. https://doi.org/10.1007/s11302-014-9415-6
Haanes KA, Edvinsson L (2014) Expression and characterization of purinergic receptors in rat middle meningeal artery-potential role in migraine. PLoS ONE 9(9):e108782. https://doi.org/10.1371/journal.pone.0108782
Mateo J, Miras-Portugal MT, Rotllán P (1997) Ecto-enzymatic hydrolysis of diadenosine polyphosphates by cultured adrenomedullary vascular endothelial cells. Am J Physiol 273(3 Pt 1):C918-927. https://doi.org/10.1152/ajpcell.1997.273.3.C918
Lee JW, Kong ID, Park KS, Jeong SW (1995) Effects of adenosine tetraphosphate (ATPP) on vascular tone in the isolated rat aorta. Yonsei Med J 36(6):487–496. https://doi.org/10.3349/ymj.1995.36.6.487
Harrington LS, Mitchell JA (2004) Novel role for P2X receptor activation in endothelium-dependent vasodilation. Br J Pharmacol 143(5):611–617. https://doi.org/10.1038/sj.bjp.0706004
Harrington LS, Evans RJ, Wray J, Norling L, Swales KE, Vial C, Ali F, Carrier MJ, Mitchell JA (2007) Purinergic 2X1 receptors mediate endothelial dependent vasodilation to ATP. Mol Pharmacol 72(5):1132–1136. https://doi.org/10.1124/mol.107.037325
Yamamoto K, Korenaga R, Kamiya A, Ando J (2000) Fluid shear stress activates Ca(2+) influx into human endothelial cells via P2X4 purinoceptors. Circ Res 87(5):385–391. https://doi.org/10.1161/01.res.87.5.385
Wang L, Karlsson L, Moses S, Hultgårdh-Nilsson A, Andersson M, Borna C, Gudbjartsson T, Jern S, Erlinge D (2002) P2 receptor expression profiles in human vascular smooth muscle and endothelial cells. J Cardiovasc Pharmacol 40(6):841–853. https://doi.org/10.1097/00005344-200212000-00005
Yamamoto K, Sokabe T, Ohura N, Nakatsuka H, Kamiya A, Ando J (2003) Endogenously released ATP mediates shear stress-induced Ca2+ influx into pulmonary artery endothelial cells. Am J Physiol Heart Circ Physiol 285(2):H793-803. https://doi.org/10.1152/ajpheart.01155.2002
Yamamoto K, Sokabe T, Matsumoto T, Yoshimura K, Shibata M, Ohura N, Fukuda T, Sato T, Sekine K, Kato S, Isshiki M, Fujita T, Kobayashi M, Kawamura K, Masuda H, Kamiya A, Ando J (2006) Impaired flow-dependent control of vascular tone and remodeling in P2X4-deficient mice. Nat Med 12(1):133–137. https://doi.org/10.1038/nm1338
Loesch A, Burnstock G (2000) Ultrastructural localisation of ATP-gated P2X2 receptor immunoreactivity in vascular endothelial cells in rat brain. Endothelium 7(2):93–98. https://doi.org/10.3109/10623320009072204
Knight GE, Oliver-Redgate R, Burnstock G (2003) Unusual absence of endothelium-dependent or -independent vasodilatation to purines or pyrimidines in the rat renal artery. Kidney Int 64(4):1389–1397. https://doi.org/10.1046/j.1523-1755.2003.00233.x
Wildman SS, King BF, Burnstock G (1998) Zn2+ modulation of ATP-responses at recombinant P2X2 receptors and its dependence on extracellular pH. Br J Pharmacol 123(6):1214–1220. https://doi.org/10.1038/sj.bjp.0701717
Ralevic V (2009) Purines as neurotransmitters and neuromodulators in blood vessels. Curr Vasc Pharmacol 7(1):3–14. https://doi.org/10.2174/157016109787354123
Chen CC, Akopian AN, Sivilotti L, Colquhoun D, Burnstock G, Wood JN (1995) A P2X purinoceptor expressed by a subset of sensory neurons. Nature 377(6548):428–431. https://doi.org/10.1038/377428a0
Zerpa H, Crawford C, Knight GE, Fordham AF, Janska SE, Peppiatt-Wildman CM, Elliott J, Burnstock G, Wildman SS (2013) Extracellular ATP signaling in equine digital blood vessels. Eur J Pharmacol 702(1–3):242–249. https://doi.org/10.1016/j.ejphar.2013.01.018
Gitterman DP, Evans RJ (2000) Properties of P2X and P2Y receptors are dependent on artery diameter in the rat mesenteric bed. Br J Pharmacol 131(8):1561–1568. https://doi.org/10.1038/sj.bjp.0703760
Jankowski J, Jankowski V, Laufer U, van der Giet M, Henning L, Tepel M, Zidek W, Schlüter H (2003) Identification and quantification of diadenosine polyphosphate concentrations in human plasma. Arterioscler Thromb Vasc Biol 23(7):1231–1238. https://doi.org/10.1161/01.ATV.0000075913.00428.FD
Phillips JK, McLean AJ, Hill CE (1998) Receptors involved in nerve-mediated vasoconstriction in small arteries of the rat hepatic mesentery. Br J Pharmacol 124(7):1403–1412. https://doi.org/10.1038/sj.bjp.0701976
Kawamura H, Sugiyama T, Wu DM, Kobayashi M, Yamanishi S, Katsumura K, Puro DG (2003) ATP: a vasoactive signal in the pericyte-containing microvasculature of the rat retina. J Physiol 551(Pt 3):787–799. https://doi.org/10.1113/jphysiol.2003.047977
Kur J, Newman EA (2014) Purinergic control of vascular tone in the retina. J Physiol 592(3):491–504. https://doi.org/10.1113/jphysiol.2013.267294
Inoue K (2021) Nociceptive signaling of P2X receptors in chronic pain states. Purinergic Signal 17(1):41–47. https://doi.org/10.1007/s11302-020-09743-w
Lewis C, Neidhart S, Holy C, North RA, Buell G, Surprenant A (1995) Coexpression of P2X2 and P2X3 receptor subunits can account for ATP-gated currents in sensory neurons. Nature 377(6548):432–435. https://doi.org/10.1038/377432a0
Lopes DM, Denk F, McMahon SB (2017) The molecular fingerprint of dorsal root and trigeminal ganglion neurons. Front Mol Neurosci 10:304. https://doi.org/10.3389/fnmol.2017.00304
Staikopoulos V, Sessle BJ, Furness JB (2007) Jennings EA (2007) Localization of P2X2 and P2X3 receptors in rat trigeminal ganglion neurons. Neuroscience 144(1):208–216. https://doi.org/10.1016/j.neuroscience.2006.09.035
Rae MG, Rowan EG, Kennedy C (1998) Pharmacological properties of P2X3-receptors present in neurones of the rat dorsal root ganglia. Br J Pharmacol 124(1):176–180. https://doi.org/10.1038/sj.bjp.0701803
Ueno S, Tsuda M, Iwanaga T, Inoue K (1999) Cell type-specific ATP-activated responses in rat dorsal root ganglion neurons. Br J Pharmacol 126(2):429–436. https://doi.org/10.1038/sj.bjp.0702319
Rodrigues RJ, Almeida T, Richardson PJ, Oliveira CR, Cunha RA (2005) Dual presynaptic control by ATP of glutamate release via facilitatory P2X1, P2X2/3, and P2X3 and inhibitory P2Y1, P2Y2, and/or P2Y4 receptors in the rat hippocampus. J Neurosci 25(27):6286–6295. https://doi.org/10.1523/JNEUROSCI.0628-05.2005
Tsuda M, Ueno S, Inoue K (1999) In vivo pathway of thermal hyperalgesia by intrathecal administration of alpha, beta-methylene ATP in mouse spinal cord: involvement of the glutamate-NMDA receptor system. Br J Pharmacol 127(2):449–456. https://doi.org/10.1038/sj.bjp.0702582
Jennings EA, Christie MJ, Sessle BJ (2006) ATP potentiates neurotransmission in the rat trigeminal subnucleus caudalis. NeuroReport 17(14):1507–1510. https://doi.org/10.1097/01.wnr.0000234740.97076.95
Cockayne DA, Dunn PM, Zhong Y, Rong W, Hamilton SG, Knight GE, Ruan HZ, Ma B, Yip P, Nunn P, McMahon SB, Burnstock G, Ford AP (2005) P2X2 knockout mice and P2X2/P2X3 double knockout mice reveal a role for the P2X2 receptor subunit in mediating multiple sensory effects of ATP. J Physiol 567(Pt 2):621–639. https://doi.org/10.1113/jphysiol.2005.088435
Long T, He W, Pan Q, Zhang S, Zhang Y, Liu C, Liu Q, Qin G, Chen L, Zhou J (2018) Microglia P2X4 receptor contributes to central sensitization following recurrent nitroglycerin stimulation. J Neuroinflammation 15(1):245. https://doi.org/10.1186/s12974-018-1285-3
Long T, He W, Pan Q, Zhang S, Zhang D, Qin G, Chen L, Zhou J (2020) Microglia P2X4R-BDNF signalling contributes to central sensitization in a recurrent nitroglycerin-induced chronic migraine model. J Headache Pain 21(1):4. https://doi.org/10.1186/s10194-019-1070-4
Jones CA, Chessell IP, Simon J, Barnard EA, Miller KJ, Michel AD, Humphrey PP (2000) Functional characterization of the P2X(4) receptor orthologues. Br J Pharmacol 129(2):388–394. https://doi.org/10.1038/sj.bjp.0703059
Gómez-Villafuertes R, Gualix J, Miras-Portugal MT, Pintor J (2000) Adenosine 5’-tetraphosphate (Ap(4)), a new agonist on rat midbrain synaptic terminal P2 receptors. Neuropharmacology 39(12):2381–2390. https://doi.org/10.1016/s0028-3908(00)00070-8
Chen L, Liu YW, Yue K, Ru Q, Xiong Q, Ma BM, Tian X, Li CY (2016) Differential expression of ATP-gated P2X receptors in DRG between chronic neuropathic pain and visceralgia rat models. Purinergic Signal 12(1):79–87. https://doi.org/10.1007/s11302-015-9481-4
Birdsong WT, Fierro L, Williams FG, Spelta V, Naves LA, Knowles M, Marsh-Haffner J, Adelman JP, Almers W, Elde RP, McCleskey EW (2010) Sensing muscle ischemia: coincident detection of acid and ATP via interplay of two ion channels. Neuron 68(4):739–749. https://doi.org/10.1016/j.neuron.2010.09.029
Burnstock G (2000) P2X receptors in sensory neurones. Br J Anaesth 84(4):476–488. https://doi.org/10.1093/oxfordjournals.bja.a013473
Bardoni R, Goldstein PA, Lee CJ, Gu JG, MacDermott AB (1997) ATP P2X receptors mediate fast synaptic transmission in the dorsal horn of the rat spinal cord. J Neurosci 17(14):5297–5304. https://doi.org/10.1523/JNEUROSCI.17-14-05297.1997
Sperlágh B, Illes P (2014) P2X7 receptor: an emerging target in central nervous system diseases. Trends Pharmacol Sci 35(10):537–547. https://doi.org/10.1016/j.tips.2014.08.002
Gölöncsér F, Sperlágh B (2014) Effect of genetic deletion and pharmacological antagonism of P2X7 receptors in a mouse animal model of migraine. J Headache Pain 15(1):24. https://doi.org/10.1186/1129-2377-15-24
D’Amico M, Samengo I, Navarra P, Taglialatela M, Martire M (2010) AMPA- and P2X7-receptor-mediated facilitation of [3H]D-aspartate release from nerve terminals isolated from the rat caudal brainstem. Neurochem Int 57(6):623–628. https://doi.org/10.1016/j.neuint.2010.07.009
Currò D, Navarra P, Samengo I, Martire M (2020) P2X7 receptors exert a permissive effect on the activation of presynaptic AMPA receptors in rat trigeminal caudal nucleus glutamatergic nerve terminals. J Headache Pain 21(1):83. https://doi.org/10.1186/s10194-020-01153-y
Jiang Y, Ye F, Du Y, Zong Y, Tang Z (2021) P2X7R in mast cells is a potential target for salicylic acid and aspirin in treatment of inflammatory pain. J Inflamm Res 14:2913–2931. https://doi.org/10.2147/JIR.S313348
Nurkhametova D, Kudryavtsev I, Guselnikova V, Serebryakova M, Giniatullina RR, Wojciechowski S, Tore F, Rizvanov A, Koistinaho J, Malm T, Giniatullin R (2019) Activation of P2X7 receptors in peritoneal and meningeal mast cells detected by uptake of organic dyes: possible purinergic triggers of neuroinflammation in meninges. Front Cell Neurosci 13:45. https://doi.org/10.3389/fncel.2019.00045
Karatas H, Erdener SE, Gursoy-Ozdemir Y, Lule S, Eren-Koçak E, Sen ZD, Dalkara T (2013) Spreading depression triggers headache by activating neuronal Panx1 channels. Science 339(6123):1092–1095. https://doi.org/10.1126/science.1231897
Chen SP, Qin T, Seidel JL, Zheng Y, Eikermann M, Ferrari MD, van den Maagdenberg AMJM, Moskowitz MA, Ayata C, Eikermann-Haerter K (2017) Inhibition of the P2X7-PANX1 complex suppresses spreading depolarization and neuroinflammation. Brain 140(6):1643–1656. https://doi.org/10.1093/brain/awx085
Chakfe Y, Seguin R, Antel JP, Morissette C, Malo D, Henderson D, Séguéla P (2002) ADP and AMP induce interleukin-1beta release from microglial cells through activation of ATP-primed P2X7 receptor channels. J Neurosci 22(8):3061–3069. https://doi.org/10.1523/JNEUROSCI.22-08-03061.2002
Abbracchio MP, Burnstock G, Boeynaems JM, Barnard EA, Boyer JL, Kennedy C, Knight GE, Fumagalli M, Gachet C, Jacobson KA, Weisman GA (2006) International Union of Pharmacology LVIII: update on the P2Y G protein-coupled nucleotide receptors: from molecular mechanisms and pathophysiology to therapy. Pharmacol Rev 58(3):281–341. https://doi.org/10.1124/pr.58.3.3
Jacobson KA (2010) P2X and P2Y receptors. Tocris Rev 33:1–16
Moore DJ, Chambers JK, Wahlin JP, Tan KB, Moore GB, Jenkins O, Emson PC, Murdock PR (2001) Expression pattern of human P2Y receptor subtypes: a quantitative reverse transcription-polymerase chain reaction study. Biochim Biophys Acta 1521(1–3):107–119. https://doi.org/10.1016/s0167-4781(01)00291-3
Waldo GL, Harden TK (2004) Agonist binding and Gq-stimulating activities of the purified human P2Y1 receptor. Mol Pharmacol 65(2):426–436. https://doi.org/10.1124/mol.65.2.426
Palmer RK, Boyer JL, Schachter JB, Nicholas RA, Harden TK (1998) Agonist action of adenosine triphosphates at the human P2Y1 receptor. Mol Pharmacol 54(6):1118–1123
Sak K, Barnard EA, Järv J (2000) Dual effect of nucleotides on P2Y receptors. IUBMB Life 50(2):99–103. https://doi.org/10.1080/713803703
Léon C, Hechler B, Vial C, Leray C, Cazenave JP, Gachet C (1997) The P2Y1 receptor is an ADP receptor antagonized by ATP and expressed in platelets and megakaryoblastic cells. FEBS Lett 403(1):26–30. https://doi.org/10.1016/s0014-5793(97)00022-7
Bourdon DM, Mahanty SK, Jacobson KA, Boyer JL, Harden TK (2006) (N)-methanocarba-2MeSADP (MRS2365) is a subtype-specific agonist that induces rapid desensitization of the P2Y1 receptor of human platelets. J Thromb Haemost 4(4):861–868. https://doi.org/10.1111/j.1538-7836.2006.01866.x
Hechler B, Vigne P, Léon C, Breittmayer JP, Gachet C, Frelin C (1998) ATP derivatives are antagonists of the P2Y1 receptor: similarities to the platelet ADP receptor. Mol Pharmacol 53(4):727–733
Webb TE, Feolde E, Vigne P, Neary JT, Runberg A, Frelin C, Barnard EA (1996) The P2Y purinoceptor in rat brain microvascular endothelial cells couple to inhibition of adenylate cyclase. Br J Pharmacol 119(7):1385–1392. https://doi.org/10.1111/j.1476-5381.1996.tb16050.x
Kauffenstein G, Fürstenau CR, D’Orléans-Juste P, Sévigny J (2010) The ecto-nucleotidase NTPDase1 differentially regulates P2Y1 and P2Y2 receptor-dependent vasorelaxation. Br J Pharmacol 159(3):576–585. https://doi.org/10.1111/j.1476-5381.2009.00566.x
Pintor J, King BF, Miras-Portugal MT, Burnstock G (1996) Selectivity and activity of adenine dinucleotides at recombinant P2X2 and P2Y1 purinoceptors. Br J Pharmacol 119(5):1006–1012. https://doi.org/10.1111/j.1476-5381.1996.tb15771.x
Chang H, Yanachkov IB, Dix EJ, Li YF, Barnard MR, Wright GE, Michelson AD, Frelinger AL 3rd (2012) Modified diadenosine tetraphosphates with dual specificity for P2Y1 and P2Y12 are potent antagonists of ADP-induced platelet activation. J Thromb Haemost 10(12):2573–2580. https://doi.org/10.1111/jth.12035
Nylander S, Mattsson C, Ramström S, Lindahl TL (2003) The relative importance of the ADP receptors, P2Y12 and P2Y1, in thrombin-induced platelet activation. Thromb Res 111(1–2):65–73. https://doi.org/10.1016/j.thromres.2003.08.021
Malin SA, Molliver DC (2010) Gi- and Gq-coupled ADP (P2Y) receptors act in opposition to modulate nociceptive signaling and inflammatory pain behavior. Mol Pain 6:21. https://doi.org/10.1186/1744-8069-6-21
Horiuchi T, Dietrich HH, Tsugane S, Dacey RG Jr (2001) Analysis of purine- and pyrimidine-induced vascular responses in the isolated rat cerebral arteriole. Am J Physiol Heart Circ Physiol 280(2):H767-776. https://doi.org/10.1152/ajpheart.2001.280.2.H767
Horiuchi T, Dietrich HH, Hongo K, Dacey RG Jr (2003) Comparison of P2 receptor subtypes producing dilation in rat intracerebral arterioles. Stroke 34(6):1473–1478. https://doi.org/10.1161/01.STR.0000071527.10129.65
Ralevic V, Burnstock G (1991) Effects of purines and pyrimidines on the rat mesenteric arterial bed. Circ Res 69(6):1583–1590. https://doi.org/10.1161/01.res.69.6.1583
Olivecrona GK, Gotberg M, Harnek J, Wang L, Jacobson KA, Erlinge D (2004) Coronary artery reperfusion: The ADP receptor P2Y(1) mediates early reactive hyperemia in vivo in pigs. Purinergic Signal 1(1):59–65. https://doi.org/10.1007/s11302-004-4742-7
Hess CN, Kou R, Johnson RP, Li GK, Michel T (2009) ADP signaling in vascular endothelial cells: ADP-dependent activation of the endothelial isoform of nitric-oxide synthase requires the expression but not the kinase activity of AMP-activated protein kinase. J Biol Chem 284(47):32209–32224. https://doi.org/10.1074/jbc.M109.032656
Bender SB, Berwick ZC, Laughlin MH (1985) Tune JD (2011) Functional contribution of P2Y1 receptors to the control of coronary blood flow. J Appl Physiol 111(6):1744–1750. https://doi.org/10.1152/japplphysiol.00946.2011
Kylhammar D, Bune LT, Rådegran G (2014) P2Y1 and P2Y12 receptors in hypoxia- and adenosine diphosphate-induced pulmonary vasoconstriction in vivo in the pig. Eur J Appl Physiol 114(9):1995–2006. https://doi.org/10.1007/s00421-014-2921-y
Mitchell R, Campbell G, Mikolajczak M, McGill K, Mahad D, Fleetwood-Walker SM (2019) A targeted mutation disrupting mitochondrial complex IV function in primary afferent neurons leads to pain hypersensitivity through P2Y1 receptor activation. Mol Neurobiol 56(8):5917–5933. https://doi.org/10.1007/s12035-018-1455-4
Barragán-Iglesias P, Pineda-Farias JB, Bravo-Hernández M, Cervantes-Durán C, Price TJ, Murbartián J, Granados-Soto V (2016) Predominant role of spinal P2Y1 receptors in the development of neuropathic pain in rats. Brain Res 1636:43–51. https://doi.org/10.1016/j.brainres.2016.01.042
Kwon SG, Roh DH, Yoon SY, Choi SR, Choi HS, Moon JY, Kang SY, Beitz AJ, Lee JH (2017) Involvement of peripheral P2Y1 receptors and potential interaction with IL-1 receptors in IL-1β-induced thermal hypersensitivity in rats. Brain Res Bull 130:165–172. https://doi.org/10.1016/j.brainresbull.2017.01.019
Schafer R, Sedehizade F, Welte T, Reiser G (2003) ATP- and UTP-activated P2Y receptors differently regulate proliferation of human lung epithelial tumor cells. Am J Physiol Lung Cell Mol Physiol 285(2):L376-385. https://doi.org/10.1152/ajplung.00447.2002
Jacobson KA, Jarvis MF, Williams M (2002) Purine and pyrimidine (P2) receptors as drug targets. J Med Chem 45(19):4057–4093. https://doi.org/10.1021/jm020046y
Kim HS, Ravi RG, Marquez VE, Maddileti S, Wihlborg AK, Erlinge D, Malmsjö M, Boyer JL, Harden TK, Jacobson KA (2002) Methanocarba modification of uracil and adenine nucleotides: high potency of Northern ring conformation at P2Y1, P2Y2, P2Y4, and P2Y11 but not P2Y6 receptors. J Med Chem 45(1):208–218. https://doi.org/10.1021/jm010369e
Lazarowski ER, Watt WC, Stutts MJ, Boucher RC, Harden TK (1995) Pharmacological selectivity of the cloned human P2U-purinoceptor: potent activation by diadenosine tetraphosphate. Br J Pharmacol 116(1):1619–1627. https://doi.org/10.1111/j.1476-5381.1995.tb16382.x
Ralevic V, Burnstock G (1998) Receptors for purines and pyrimidines. Pharmacol Rev 50(3):413–492
Boarder MR, Weisman GA, Turner JT, Wilkinson GF (1995) G protein-coupled P2 purinoceptors: from molecular biology to functional responses. Trends Pharmacol Sci 16(4):133–139. https://doi.org/10.1016/s0165-6147(00)89001-x
Shihan M, Novoyatleva T, Lehmeyer T, Sydykov A, Schermuly RT (2021) Role of the purinergic P2Y2 receptor in pulmonary hypertension. Int J Environ Res Public Health 18(21):11009. https://doi.org/10.3390/ijerph182111009
Alvarado-Castillo C, Harden TK, Boyer JL (2005) Regulation of P2Y1 receptor-mediated signaling by the ectonucleoside triphosphate diphosphohydrolase isozymes NTPDase1 and NTPDase2. Mol Pharmacol 67(1):114–122. https://doi.org/10.1124/mol.104.006908
Guns PJ, Korda A, Crauwels HM, Van Assche T, Robaye B, Boeynaems JM, Bult H (2005) Pharmacological characterization of nucleotide P2Y receptors on endothelial cells of the mouse aorta. Br J Pharmacol 146(2):288–295. https://doi.org/10.1038/sj.bjp.0706326
Wang S, Iring A, Strilic B, Albarrán Juárez J, Kaur H, Troidl K, Tonack S, Burbiel JC, Müller CE, Fleming I, Lundberg JO, Wettschureck N, Offermanns S (2015) P2Y2 and Gq/G11 control blood pressure by mediating endothelial mechanotransduction. J Clin Invest 125(8):3077–3086. https://doi.org/10.1172/JCI81067
Molliver DC, Cook SP, Carlsten JA, Wright DE, McCleskey EW (2002) ATP and UTP excite sensory neurons and induce CREB phosphorylation through the metabotropic receptor, P2Y2. Eur J Neurosci 16(10):1850–1860. https://doi.org/10.1046/j.1460-9568.2002.02253.x
Niederberger E, Ehnert C, Gao W, Coste O, Schmidtko A, Popp L, Gall CV, Korf HW, Tegeder I, Geisslinger G (2007) The impact of CREB and its phosphorylation at Ser142 on inflammatory nociception. Biochem Biophys Res Commun 362(1):75–80. https://doi.org/10.1016/j.bbrc.2007.07.148
Malin SA, Davis BM, Koerber RH, Reynolds IJ, Albers KM, Molliver DC (2008) Thermal nociception and TRPV1 function are attenuated in mice lacking the nucleotide receptor P2Y2. Pain 138(3):484–496. https://doi.org/10.1016/j.pain.2008.01.026
Magni G, Merli D, Verderio C, Abbracchio MP, Ceruti S (2015) P2Y2 receptor antagonists as anti-allodynic agents in acute and sub-chronic trigeminal sensitization: role of satellite glial cells. Glia 63(7):1256–1269. https://doi.org/10.1002/glia.22819
Communi D, Robaye B, Boeynaems JM (1999) Pharmacological characterization of the human P2Y11 receptor. Br J Pharmacol 128(6):1199–1206. https://doi.org/10.1038/sj.bjp.0702909
Invitrogen (2008). GeneBLAzer® validation Packet (Version No.: 01Sep08). https://tools.thermofisher.com/content/sfs/manuals/P2RY11-DA-and-D.pdf
White PJ, Webb TE, Boarder MR (2003) Characterization of a Ca2+ response to both UTP and ATP at human P2Y11 receptors: evidence for agonist-specific signaling. Mol Pharmacol 63(6):1356–1363. https://doi.org/10.1124/mol.63.6.1356
van der Weyden L, Adams DJ, Luttrell BM, Conigrave AD, Morris MB (2000) Pharmacological characterisation of the P2Y11 receptor in stably transfected haematological cell lines. Mol Cell Biochem 213(1–2):75–81. https://doi.org/10.1023/a:1007168215748
Qi AD, Zambon AC, Insel PA, Nicholas RA (2001) An arginine/glutamine difference at the juxtaposition of transmembrane domain 6 and the third extracellular loop contributes to the markedly different nucleotide selectivities of human and canine P2Y11 receptors. Mol Pharmacol 60(6):1375–1382. https://doi.org/10.1124/mol.60.6.1375
van der Weyden L, Conigrave AD, Morris MB (2000) Signal transduction and white cell maturation via extracellular ATP and the P2Y11 receptor. Immunol Cell Biol 78(4):369–374. https://doi.org/10.1046/j.1440-1711.2000.00918.x
Liu C, Mather S, Huang Y, Garland CJ, Yao X (2004) Extracellular ATP facilitates flow-induced vasodilatation in rat small mesenteric arteries. Am J Physiol Heart Circ Physiol 286(5):H1688-1695. https://doi.org/10.1152/ajpheart.00576.2003
Dănilă MD, Privistirescu A, Duicu OM, Rațiu CD, Angoulvant D, Muntean DM, Sturza A (2017) The effect of purinergic signaling via the P2Y11 receptor on vascular function in a rat model of acute inflammation. Mol Cell Biochem 431(1–2):37–44. https://doi.org/10.1007/s11010-017-2973-5
Piollet M, Sturza A, Chadet S, Gabillard-Lefort C, Benoist L, Muntean DM, Aburel OM, Angoulvant D, Ivanes F (2021) P2Y11 agonism prevents hypoxia/reoxygenation- and angiotensin ii-induced vascular dysfunction and intimal hyperplasia development. Int J Mol Sci 22(2):855. https://doi.org/10.3390/ijms22020855
Barragán-Iglesias P, Pineda-Farias JB, Cervantes-Durán C, Bravo-Hernández M, Rocha-González HI, Murbartián J, Granados-Soto V (2014) Role of spinal P2Y6 and P2Y11 receptors in neuropathic pain in rats: possible involvement of glial cells. Mol Pain 10:29. https://doi.org/10.1186/1744-8069-10-29
Barragán-Iglesias P, Mendoza-Garcés L, Pineda-Farias JB, Solano-Olivares V, Rodríguez-Silverio J, Flores-Murrieta FJ, Granados-Soto V, Rocha-González HI (2015) Participation of peripheral P2Y1, P2Y6 and P2Y11 receptors in formalin-induced inflammatory pain in rats. Pharmacol Biochem Behav 128:23–32. https://doi.org/10.1016/j.pbb.2014.11.001
Simon J, Filippov AK, Göransson S, Wong YH, Frelin C, Michel AD, Brown DA, Barnard EA (2002) Characterization and channel coupling of the P2Y(12) nucleotide receptor of brain capillary endothelial cells. J Biol Chem 277(35):31390–31400. https://doi.org/10.1074/jbc.M110714200
Ding Z, Kim S, Dorsam RT, Jin J, Kunapuli SP (2003) Inactivation of the human P2Y12 receptor by thiol reagents requires interaction with both extracellular cysteine residues, Cys17 and Cys270. Blood 101(10):3908–3914. https://doi.org/10.1182/blood-2002-10-3027
Bodor ET, Waldo GL, Hooks SB, Corbitt J, Boyer JL, Harden TK (2003) Purification and functional reconstitution of the human P2Y12 receptor. Mol Pharmacol 64(5):1210–1216. https://doi.org/10.1124/mol.64.5.1210
Zhang FL, Luo L, Gustafson E, Palmer K, Qiao X, Fan X, Yang S, Laz TM, Bayne M, Monsma F Jr (2002) P2Y(13): identification and characterization of a novel Galphai-coupled ADP receptor from human and mouse. J Pharmacol Exp Ther 301(2):705–713. https://doi.org/10.1124/jpet.301.2.705
Quinton TM, Kim S, Dangelmaier C, Dorsam RT, Jin J, Daniel JL, Kunapuli SP (2002) Protein kinase C- and calcium-regulated pathways independently synergize with Gi pathways in agonist-induced fibrinogen receptor activation. Biochem J 368(Pt 2):535–543. https://doi.org/10.1042/BJ20020226
Dorsam RT, Kim S, Jin J, Kunapuli SP (2002) Coordinated signaling through both G12/13 and G(i) pathways is sufficient to activate GPIIb/IIIa in human platelets. J Biol Chem 277(49):47588–47595. https://doi.org/10.1074/jbc.M208778200
Ho MK, Wong YH (2001) G(z) signaling: emerging divergence from G(i) signaling. Oncogene 20(13):1615–1625. https://doi.org/10.1038/sj.onc.1204190
Wihlborg AK, Wang L, Braun OO, Eyjolfsson A, Gustafsson R, Gudbjartsson T, Erlinge D (2004) ADP receptor P2Y12 is expressed in vascular smooth muscle cells and stimulates contraction in human blood vessels. Arterioscler Thromb Vasc Biol 24(10):1810–1815. https://doi.org/10.1161/01.ATV.0000142376.30582.ed
Rey M, Kramberg M, Hess P, Morrison K, Ernst R, Haag F, Weber E, Clozel M, Baumann M, Caroff E, Hubler F, Riederer MA, Steiner B (2017) The reversible P2Y12 antagonist ACT-246475 causes significantly less blood loss than ticagrelor at equivalent antithrombotic efficacy in rat. Pharmacol Res Perspect 5(5):e00338. https://doi.org/10.1002/prp2.338
Skyhøj Olsen T (1990) Migraine with and without aura: the same disease due to cerebral vasospasm of different intensity. A hypothesis based on CBF studies during migraine. Headache 30(5):269–272. https://doi.org/10.1111/j.1526-4610.1990.hed3005269.x
Gölöncsér F, Baranyi M, Iring A, Hricisák L, Otrokocsi L, Benyó Z, Sperlágh B (2021) Involvement of P2Y12 receptors in a nitroglycerin-induced model of migraine in male mice. Br J Pharmacol 178(23):4626–4645. https://doi.org/10.1111/bph.15641
Jing F, Zhang Y, Long T, He W, Qin G, Zhang D, Chen L, Zhou J (2019) P2Y12 receptor mediates microglial activation via RhoA/ROCK pathway in the trigeminal nucleus caudalis in a mouse model of chronic migraine. J Neuroinflammation 16(1):217. https://doi.org/10.1186/s12974-019-1603-4
Yu T, Zhang X, Shi H, Tian J, Sun L, Hu X, Cui W, Du D (2019) P2Y12 regulates microglia activation and excitatory synaptic transmission in spinal lamina II neurons during neuropathic pain in rodents. Cell Death Dis 10(3):165. https://doi.org/10.1038/s41419-019-1425-4
Kobayashi K, Yamanaka H, Fukuoka T, Dai Y, Obata K, Noguchi K (2008) P2Y12 receptor upregulation in activated microglia is a gateway of p38 signaling and neuropathic pain. J Neurosci 28(11):2892–2902. https://doi.org/10.1523/JNEUROSCI.5589-07.2008
Tsuchida R, Sumitani M, Abe H, Ando M, Saita K, Hattori K, Mietani K, Inoue R, Uchida K (2020) Clopidogrel, an ADP-P2Y12 receptor antagonist, can prevent severe postoperative pain: a retrospective chart review. Life (Basel) 10(6):92. https://doi.org/10.3390/life10060092
Liu PW, Yue MX, Zhou R, Niu J, Huang DJ, Xu T, Luo P, Liu XH, Zeng JW (2017) P2Y12 and P2Y13 receptors involved in ADPβs induced the release of IL-1β, IL-6 and TNF-α from cultured dorsal horn microglia. J Pain Res 10:1755–1767. https://doi.org/10.2147/JPR.S137131
Perini F, D’Andrea G, Galloni E, Pignatelli F, Billo G, Alba S, Bussone G, Toso V (2005) Plasma cytokine levels in migraineurs and controls. Headache 45(7):926–931. https://doi.org/10.1111/j.1526-4610.2005.05135.x
Koçer A, Memişoğullari R, Domaç FM, Ilhan A, Koçer E, Okuyucu S, Ozdemir B, Yüksel H (2009) IL-6 levels in migraine patients receiving topiramate. Pain Pract 9(5):375–379. https://doi.org/10.1111/j.1533-2500.2009.00301.x
Marteau F, Le Poul E, Communi D, Communi D, Labouret C, Savi P, Boeynaems JM, Gonzalez NS (2003) Pharmacological characterization of the human P2Y13 receptor. Mol Pharmacol 64(1):104–112. https://doi.org/10.1124/mol.64.1.104
Communi D, Gonzalez NS, Detheux M, Brézillon S, Lannoy V, Parmentier M, Boeynaems JM (2001) Identification of a novel human ADP receptor coupled to G(i). J Biol Chem 276(44):41479–41485. https://doi.org/10.1074/jbc.M105912200
Kim YC, Lee JS, Sak K, Marteau F, Mamedova L, Boeynaems JM, Jacobson KA (2005) Synthesis of pyridoxal phosphate derivatives with antagonist activity at the P2Y13 receptor. Biochem Pharmacol 70(2):266–274. https://doi.org/10.1016/j.bcp.2005.04.021
Dsouza C, Komarova SV (2021) Characterization of potency of the P2Y13 receptor agonists: a meta-analysis. Int J Mol Sci 22(7):3468. https://doi.org/10.3390/ijms22073468
Giachini FR, Leite R, Osmond DA, Lima VV, Inscho EW, Webb RC, Tostes RC (2014) Anti-platelet therapy with clopidogrel prevents endothelial dysfunction and vascular remodeling in aortas from hypertensive rats. PLoS ONE 9(3):e91890. https://doi.org/10.1371/journal.pone.0091890
Zhou R, Xu T, Liu X, Chen Y, Kong D, Tian H, Yue M, Huang D, Zeng J (2018) Activation of spinal dorsal horn P2Y13 receptors can promote the expression of IL-1β and IL-6 in rats with diabetic neuropathic pain. J Pain Res 11:615–628. https://doi.org/10.2147/JPR.S154437
Gao N, Hu HZ, Liu S, Gao C, Xia Y, Wood JD (2007) Stimulation of adenosine A1 and A2A receptors by AMP in the submucosal plexus of guinea pig small intestine. Am J Physiol Gastrointest Liver Physiol 292(2):G492-500. https://doi.org/10.1152/ajpgi.00257.2006
Mustafa SJ, Nadeem A, Fan M, Zhong H, Belardinelli L, Zeng D (2007) Effect of a specific and selective A(2B) adenosine receptor antagonist on adenosine agonist AMP and allergen-induced airway responsiveness and cellular influx in a mouse model of asthma. J Pharmacol Exp Ther 320(3):1246–1251. https://doi.org/10.1124/jpet.106.112250
Holien JK, Seibt B, Roberts V, Salvaris E, Parker MW, Cowan PJ, Dwyer KM (2018) AMP and adenosine are both ligands for adenosine 2B receptor signaling. Bioorg Med Chem Lett 28(2):202–206. https://doi.org/10.1016/j.bmcl.2017.11.019
Rittiner JE, Korboukh I, Hull-Ryde EA, Jin J, Janzen WP, Frye SV, Zylka MJ (2012) AMP is an adenosine A1 receptor agonist. J Biol Chem 287(8):5301–5309. https://doi.org/10.1074/jbc.M111.291666
Gualix J, Abal M, Pintor J, Miras-Portugal MT (1996) Presence of epsilon-adenosine tetraphosphate in chromaffin granules after transport of epsilon-ATP. FEBS Lett 391(1–2):195–198. https://doi.org/10.1016/0014-5793(96)00732-6
Van Dyke K, Robinson R, Urquilla P, Smith D, Taylor M, Trush M, Wilson M (1977) An analysis of nucleotides and catecholamines in bovine medullary granules by anion exchange high pressure liquid chromatography and fluorescence. Evidence that most of the catecholamines in chromaffin granules are stored without associated ATP. Pharmacology 15(5):377–391. https://doi.org/10.1159/000136714
Westhoff T, Jankowski J, Schmidt S, Luo J, Giebing G, Schlüter H, Tepel M, Zidek W, van der Giet M (2003) Identification and characterization of adenosine 5’-tetraphosphate in human myocardial tissue. J Biol Chem 278(20):17735–17740. https://doi.org/10.1074/jbc.M300288200
Hourani SM, Bailey SJ, Johnson CR, Tennant JP (1998) Effects of adenosine 5’-triphosphate, uridine 5’-triphosphate, adenosine 5’-tetraphosphate and diadenosine polyphosphates in guinea-pig taenia caeci and rat colon muscularis mucosae. Naunyn Schmiedebergs Arch Pharmacol 358(4):464–473. https://doi.org/10.1007/pl00005279
Miras-Portugal MT, Gualix J, Pintor J (1998) The neurotransmitter role of diadenosine polyphosphates. FEBS Lett 430(1–2):78–82. https://doi.org/10.1016/s0014-5793(98)00560-2
Lüthje J, Ogilvie A (1983) The presence of diadenosine 5’,5’’’-P1, P3-triphosphate (Ap3A) in human platelets. Biochem Biophys Res Commun 115(1):253–260. https://doi.org/10.1016/0006-291x(83)90997-x
Schlüter H, Offers E, Brüggemann G, van der Giet M, Tepel M, Nordhoff E, Karas M, Spieker C, Witzel H, Zidek W (1994) Diadenosine phosphates and the physiological control of blood pressure. Nature 367(6459):186–188. https://doi.org/10.1038/367186a0
Schlüter H, Tepel M, Zidek W (1996) Vascular actions of diadenosine phosphates. J Auton Pharmacol 16(6):357–362. https://doi.org/10.1111/j.1474-8673.1996.tb00053.x
Schulze-Lohoff E, Zanner S, Ogilvie A, Sterzel RB (1995) Vasoactive diadenosine polyphosphates promote growth of cultured renal mesangial cells. Hypertension 26(6 Pt 1):899–904. https://doi.org/10.1161/01.hyp.26.6.899
Jankowski J, Tepel M, van der Giet M, Tente IM, Henning L, Junker R, Zidek W, Schlüter H (1999) Identification and characterization of P(1), P(7)-Di(adenosine-5’)-heptaphosphate from human platelets. J Biol Chem 274(34):23926–23931. https://doi.org/10.1074/jbc.274.34.23926
Rodriguez del Castillo A, Torres M, Delicado EG, Miras-Portugal MT (1988) Subcellular distribution studies of diadenosine polyphosphates—Ap4A and Ap5A—in bovine adrenal medulla: presence in chromaffin granules. J Neurochem 51(6):1696–1703. https://doi.org/10.1111/j.1471-4159.1988.tb01147.x
Pintor J, Rotllán P, Torres M, Miras-Portugal MT (1992) Characterization and quantification of diadenosine hexaphosphate in chromaffin cells: granular storage and secretagogue-induced release. Anal Biochem 200(2):296–300. https://doi.org/10.1016/0003-2697(92)90469-n
Pintor J, Puche JA, Gualix J, Hoyle CH, Miras-Portugal MT (1997) Diadenosine polyphosphates evoke Ca2+ transients in guinea-pig brain via receptors distinct from those for ATP. J Physiol 504(Pt 2):327–335. https://doi.org/10.1111/j.1469-7793.1997.327be.x
Jovanovic A, Alekseev AE, Terzic A (1997) Intracellular diadenosine polyphosphates: a novel family of inhibitory ligands of the ATP-sensitive K+ channel. Biochem Pharmacol 54(2):219–225. https://doi.org/10.1016/s0006-2952(97)00262-1
Jovanovic A, Terzic A (1996) Diadenosine tetraphosphate-induced inhibition of ATP-sensitive K+ channels in patches excised from ventricular myocytes. Br J Pharmacol 117(2):233–235. https://doi.org/10.1111/j.1476-5381.1996.tb15180.x
Ripoll C, Martin F, Manuel Rovira J, Pintor J, Miras-Portugal MT, Soria B (1996) Diadenosine polyphosphates. A novel class of glucose-induced intracellular messengers in the pancreatic beta-cell. Diabetes 45(10):1431–1434. https://doi.org/10.2337/diab.45.10.1431
Rapaport E, Zamecnik PC (1976) Presence of diadenosine 5’,5’" -P1, P4-tetraphosphate (Ap4A) in mammalian cells in levels varying widely with proliferative activity of the tissue: a possible positive “pleiotypic activator.” Proc Natl Acad Sci U S A 73(11):3984–3988. https://doi.org/10.1073/pnas.73.11.3984
Winther K, Hedman C, Flodgaard H (1990) Platelet P1, P4-Di (adenosine-51) tetraphosphate (AP4A) in migraine patients before and during beta-adrenoceptor blockade. Eur J Clin Invest 20(3):336–338. https://doi.org/10.1111/j.1365-2362.1990.tb01866.x
Gawel M, Burkitt M, Rose FC (1979) The platelet release reaction during migraine attacks. Headache 19(6):323–327. https://doi.org/10.1111/j.1526-4610.1979.hed1906323.x
Sharda A, Flaumenhaft R (2018) The life cycle of platelet granules. F1000Res 7:236. https://doi.org/10.12688/f1000research.13283.1
Chang H, Yanachkov IB, Michelson AD, Li Y, Barnard MR, Wright GE, Frelinger AL 3rd (2010) Agonist and antagonist effects of diadenosine tetraphosphate, a platelet dense granule constituent, on platelet P2Y1, P2Y12 and P2X1 receptors. Thromb Res 125(2):159–165. https://doi.org/10.1016/j.thromres.2009.11.006
Wildman SS, Brown SG, Rahman M, Noel CA, Churchill L, Burnstock G, Unwin RJ, King BF (2002) Sensitization by extracellular Ca(2+) of rat P2X(5) receptor and its pharmacological properties compared with rat P2X(1). Mol Pharmacol 62(4):957–966. https://doi.org/10.1124/mol.62.4.957
Wildman SS, Brown SG, King BF, Burnstock G (1999) Selectivity of diadenosine polyphosphates for rat P2X receptor subunits. Eur J Pharmacol 367(1):119–123. https://doi.org/10.1016/s0014-2999(98)00976-5
Shapiro MJ, Jellinek M, Pyrros D, Sundine M, Baue AE (1992) Clearance and maintenance of blood nucleotide levels with adenosine triphosphate-magnesium chloride injection. Circ Shock 36(1):62–67
Iwata K, Haruki S, Kimura T (1995) High-performance liquid chromatographic determination of diadenosine 5’,5’"-p1, p4-tetraphosphate with precolumn fluorescence derivatization and its application to metabolism study in whole blood. J Chromatogr B Biomed Appl 667(2):339–343. https://doi.org/10.1016/0378-4347(95)00042-h
Coade SB, Pearson JD (1989) Metabolism of adenine nucleotides in human blood. Circ Res 65(3):531–537. https://doi.org/10.1161/01.res.65.3.531
Busse R, Ogilvie A, Pohl U (1988) Vasomotor activity of diadenosine triphosphate and diadenosine tetraphosphate in isolated arteries. Am J Physiol 254(5 Pt 2):H828-832. https://doi.org/10.1152/ajpheart.1988.254.5.H828
Steinmetz M, Janssen AK, Pelster F, Rahn KH, Schlatter E (2002) Vasoactivity of diadenosine polyphosphates in human small mesenteric resistance arteries. J Pharmacol Exp Ther 302(2):787–794. https://doi.org/10.1124/jpet.302.2.787
Boyer JL, Romero-Avila T, Schachter JB, Harden TK (1996) Identification of competitive antagonists of the P2Y1 receptor. Mol Pharmacol 50(5):1323–1329
Steinmetz M, Gabriëls G, Le TV, Piechota HJ, Rahn KH, Schlatter E (2003) Vasoactivity of diadenosine polyphosphates in human small renal resistance arteries. Nephrol Dial Transplant 18(12):2496–2504. https://doi.org/10.1093/ndt/gfg405
Conant AR, Fisher MJ, McLennan AG, Simpson AW (2000) Diadenosine polyphosphates are largely ineffective as agonists at natively expressed P2Y(1) and P2Y(2) receptors on cultured human saphenous vein endothelial cells. J Vasc Res 37(6):548–555. https://doi.org/10.1159/000054088
Pintor J, Díaz-Hernández M, Gualix J, Gómez-Villafuertes R, Hernando F, Miras-Portugal MT (2000) Diadenosine polyphosphate receptors from rat and guinea-pig brain to human nervous system. Pharmacol Ther 87(2–3):103–115. https://doi.org/10.1016/s0163-7258(00)00049-8
Pintor J, Díaz-Hernández M, Bustamante C, Gualix J, de Terreros FJ, Miras-Portugal MT (1999) Presence of dinucleotide and ATP receptors in human cerebrocortical synaptic terminals. Eur J Pharmacol 366(2–3):159–165. https://doi.org/10.1016/s0014-2999(98)00922-4
Pintor J, Miras-Portugal MT (1995) A novel receptor for diadenosine polyphosphates coupled to calcium increase in rat midbrain synaptosomes. Br J Pharmacol 115(6):895–902. https://doi.org/10.1111/j.1476-5381.1995.tb15894.x
Burgstahler R, Grafe P (2001) Diadenosine pentaphosphate is more potent than ATP at P2X receptors in isolated rat vagus nerve. NeuroReport 12(4):679–682. https://doi.org/10.1097/00001756-200103260-00012
Trezise DJ, Michel AD, Grahames CB, Khakh BS, Surprenant A, Humphrey PP (1995) The selective P2X purinoceptor agonist, beta, gamma-methylene-L-adenosine 5’-triphosphate, discriminates between smooth muscle and neuronal P2X purinoceptors. Naunyn Schmiedebergs Arch Pharmacol 351(6):603–609. https://doi.org/10.1007/BF00170159
Viatchenko-Karpinski V, Novosolova N, Ishchenko Y, Azhar MA, Wright M, Tsintsadze V, Kamal A, Burnashev N, Miller AD, Voitenko N, Giniatullin R, Lozovaya N (2016) Stable, synthetic analogs of diadenosine tetraphosphate inhibit rat and human P2X3 receptors and inflammatory pain. Mol Pain 12:1744806916637704. https://doi.org/10.1177/1744806916637704
Klishin A, Lozovaya N, Pintor J, Miras-Portugal MT, Krishtal O (1994) Possible functional role of diadenosine polyphosphates: negative feedback for excitation in hippocampus. Neuroscience 58(2):235–236. https://doi.org/10.1016/0306-4522(94)90030-2
Heistad DD, Marcus ML, Gourley JK, Busija DW (1981) Effect of adenosine and dipyridamole on cerebral blood flow. Am J Physiol 240(5):H775-780. https://doi.org/10.1152/ajpheart.1981.240.5.H775
Shaw S, Uniyal A, Gadepalli A, Tiwari V, Belinskaia DA, Shestakova NN, Venugopala KN, Deb PK, Tiwari V (2020) Adenosine receptor signalling: probing the potential pathways for the ministration of neuropathic pain. Eur J Pharmacol 889:173619. https://doi.org/10.1016/j.ejphar.2020.173619
Sawynok J (2016) Adenosine receptor targets for pain. Neuroscience 338:1–18. https://doi.org/10.1016/j.neuroscience.2015.10.031
Vincenzi F, Pasquini S, Borea PA, Varani K (2020) Targeting adenosine receptors: a potential pharmacological avenue for acute and chronic pain. Int J Mol Sci 21(22):8710. https://doi.org/10.3390/ijms21228710
Chen JF, Eltzschig HK, Fredholm BB (2013) Adenosine receptors as drug targets—what are the challenges? Nat Rev Drug Discov 12(4):265–286. https://doi.org/10.1038/nrd3955
Amouzadeh HR, Dimery I, Werner J, Ngarmchamnanrith G, Engwall MJ, Vargas HM, Arrindell D (2019) Clinical implications and translation of an off-target pharmacology profiling hit: adenosine uptake inhibition in vitro. Transl Oncol 12(10):1296–1304. https://doi.org/10.1016/j.tranon.2019.05.018
Kruuse C, Lassen LH, Iversen HK, Oestergaard S, Olesen J (2006) Dipyridamole may induce migraine in patients with migraine without aura. Cephalalgia 26(8):925–933. https://doi.org/10.1111/j.1468-2982.2006.01137.x
Kruuse C, Thomsen LL, Jacobsen TB, Olesen J (2002) The phosphodiesterase 5 inhibitor sildenafil has no effect on cerebral blood flow or blood velocity, but nevertheless induces headache in healthy subjects. J Cereb Blood Flow Metab 22(9):1124–1131. https://doi.org/10.1097/00004647-200209000-00010
Sollevi A, Ericson K, Eriksson L, Lindqvist C, Lagerkranser M, Stone-Elander S (1987) Effect of adenosine on human cerebral blood flow as determined by positron emission tomography. J Cereb Blood Flow Metab 7(6):673–678. https://doi.org/10.1038/jcbfm.1987.121
Soricelli A, Postiglione A, Cuocolo A, De Chiara S, Ruocco A, Brunetti A, Salvatore M, Ell PJ (1995) Effect of adenosine on cerebral blood flow as evaluated by single-photon emission computed tomography in normal subjects and in patients with occlusive carotid disease. A comparison with acetazolamide Stroke 26(9):1572–1576. https://doi.org/10.1161/01.str.26.9.1572
Yarbrough GG, McGuffin-Clineschmidt JC (1981) In vivo behavioral assessment of central nervous system purinergic receptors. Eur J Pharmacol 76(2–3):137–144. https://doi.org/10.1016/0014-2999(81)90495-7
Sawynok J, Sweeney MI, White TD (1986) Classification of adenosine receptors mediating antinociception in the rat spinal cord. Br J Pharmacol 88(4):923–930. https://doi.org/10.1111/j.1476-5381.1986.tb16267.x
Goadsby PJ, Hoskin KL, Storer RJ, Edvinsson L, Connor HE (2002) Adenosine A1 receptor agonists inhibit trigeminovascular nociceptive transmission. Brain 125(Pt 6):1392–1401. https://doi.org/10.1093/brain/awf141
Yoon MH, Bae HB, Choi JI (2005) Antinociception of intrathecal adenosine receptor subtype agonists in rat formalin test. Anesth Analg 101(5):1417–1421. https://doi.org/10.1213/01.ANE.0000180994.10087.6F
Rane K, Segerdahl M, Goiny M, Sollevi A (1998) Intrathecal adenosine administration: a phase 1 clinical safety study in healthy volunteers, with additional evaluation of its influence on sensory thresholds and experimental pain. Anesthesiology 89(5):1108–1115. https://doi.org/10.1097/00000542-199811000-00010
Rauck RL, North J, Eisenach JC (2015) Intrathecal clonidine and adenosine: effects on pain and sensory processing in patients with chronic regional pain syndrome. Pain 156(1):88–95. https://doi.org/10.1016/j.pain.0000000000000007
Curto M, Lionetto L, Negro A, Capi M, Fazio F, Giamberardino MA, Simmaco M, Nicoletti F, Martelletti P (2016) Altered kynurenine pathway metabolites in serum of chronic migraine patients. J Headache Pain 17:47. https://doi.org/10.1186/s10194-016-0638-5
Liu YJ, Chen J, Li X, Zhou X, Hu YM, Chu SF, Peng Y, Chen NH (2019) Research progress on adenosine in central nervous system diseases. CNS Neurosci Ther 25(9):899–910. https://doi.org/10.1111/cns.13190
Fredholm BB, IJzerman AP, Jacobson KA, Linden J, Müller CE (2011) International Union of Basic and Clinical Pharmacology. LXXXI. Nomenclature and classification of adenosine receptors—an update. Pharmacol 63(1):1–34. https://doi.org/10.1124/pr.110.003285
Biber K, Klotz KN, Berger M, Gebicke-Härter PJ, van Calker D (1997) Adenosine A1 receptor-mediated activation of phospholipase C in cultured astrocytes depends on the level of receptor expression. J Neurosci 17(13):4956–4964. https://doi.org/10.1523/JNEUROSCI.17-13-04956.1997
Gessi S, Merighi S, Varani K, Leung E, Mac Lennan S, Borea PA (2008) The A3 adenosine receptor: an enigmatic player in cell biology. Pharmacol Ther 117(1):123–140. https://doi.org/10.1016/j.pharmthera.2007.09.002
Gao ZG, Inoue A, Jacobson KA (2018) On the G protein-coupling selectivity of the native A2B adenosine receptor. Biochem Pharmacol 151:201–213. https://doi.org/10.1016/j.bcp.2017.12.003
Feoktistov I, Goldstein AE, Ryzhov S, Zeng D, Belardinelli L, Voyno-Yasenetskaya T, Biaggioni I (2002) Differential expression of adenosine receptors in human endothelial cells: role of A2B receptors in angiogenic factor regulation. Circ Res 90(5):531–538. https://doi.org/10.1161/01.res.0000012203.21416.14
Sheth S, Brito R, Mukherjea D, Rybak LP, Ramkumar V (2014) Adenosine receptors: expression, function and regulation. Int J Mol Sci 15(2):2024–2052. https://doi.org/10.3390/ijms15022024
Cunha RA (2001) Adenosine as a neuromodulator and as a homeostatic regulator in the nervous system: different roles, different sources and different receptors. Neurochem Int 38(2):107–125. https://doi.org/10.1016/s0197-0186(00)00034-6
Jm Li, Fenton RA, Wheeler HB, Powell CC, Peyton BD, Cutler BS, Dobson JG Jr (1998) Adenosine A2a receptors increase arterial endothelial cell nitric oxide. J Surg Res 80(2):357–364. https://doi.org/10.1006/jsre.1998.5439
Hansen PB, Hashimoto S, Oppermann M, Huang Y, Briggs JP, Schnermann J (2005) Vasoconstrictor and vasodilator effects of adenosine in the mouse kidney due to preferential activation of A1 or A2 adenosine receptors. J Pharmacol Exp Ther 315(3):1150–1157. https://doi.org/10.1124/jpet.105.091017
Ray CJ, Abbas MR, Coney AM, Marshall JM (2002) Interactions of adenosine, prostaglandins and nitric oxide in hypoxia-induced vasodilatation: in vivo and in vitro studies. J Physiol 544(Pt 1):195–209. https://doi.org/10.1113/jphysiol.2002.023440
Ray CJ, Marshall JM (2006) The cellular mechanisms by which adenosine evokes release of nitric oxide from rat aortic endothelium. J Physiol 570(Pt 1):85–96. https://doi.org/10.1113/jphysiol.2005.099390
Ikeda U, Kurosaki K, Ohya K, Shimada K (1997) Adenosine stimulates nitric oxide synthesis in vascular smooth muscle cells. Cardiovasc Res 35(1):168–174. https://doi.org/10.1016/s0008-6363(97)00068-0
Olanrewaju HA, Mustafa SJ (2000) Adenosine A(2A) and A(2B) receptors mediated nitric oxide production in coronary artery endothelial cells. Gen Pharmacol 35(3):171–177. https://doi.org/10.1016/s0306-3623(01)00107-0
Dellabianca A, Faniglione M, De Angelis S, Tonini S, Balestra B, Colucci M, Cervio M, Clavenzani P, Chiocchetti R, De Giorgio R, Candura SM (2009) Adenosine A1 and A3 receptor agonists inhibit nonadrenergic, noncholinergic relaxations in the guinea pig isolated trachea. Respiration 78(1):75–83. https://doi.org/10.1159/000183755
Maddock HL, Broadley KJ, Bril A, Khandoudi N (2002) Effects of adenosine receptor agonists on guinea-pig isolated working hearts and the role of endothelium and NO. J Pharm Pharmacol 54(6):859–867. https://doi.org/10.1211/0022357021779041
Raddant AC, Russo AF (2011) Calcitonin gene-related peptide in migraine: intersection of peripheral inflammation and central modulation. Expert Rev Mol Med 13:e36. https://doi.org/10.1017/S1462399411002067
Lu W, Li B, Chen J, Su Y, Dong X, Su X, Gao L (2016) Expression of calcitonin gene-related peptide, adenosine A2a receptor and adenosine A1 receptor in experiment rat migraine models. Biomed Rep 4(3):379–383. https://doi.org/10.3892/br.2016.591
Kan HW, Chang CH, Lin CL, Lee YC, Hsieh ST, Hsieh YL (2018) Downregulation of adenosine and adenosine A1 receptor contributes to neuropathic pain in resiniferatoxin neuropathy. Pain 159(8):1580–1591. https://doi.org/10.1097/j.pain.0000000000001246
Fried NT, Elliott MB, Oshinsky ML (2017) The role of adenosine signaling in headache: a review. Brain Sci 7(3):30. https://doi.org/10.3390/brainsci7030030
Sebastião AM, Macedo MP, Ribeiro JA (2000) Tonic activation of A(2A) adenosine receptors unmasks, and of A(1) receptors prevents, a facilitatory action of calcitonin gene-related peptide in the rat hippocampus. Br J Pharmacol 129(2):374–380. https://doi.org/10.1038/sj.bjp.0703048
Ferré S, Diamond I, Goldberg SR, Yao L, Hourani SM, Huang ZL, Urade Y, Kitchen I (2007) Adenosine A2A receptors in ventral striatum, hypothalamus and nociceptive circuitry implications for drug addiction, sleep and pain. Prog Neurobiol 83(5):332–347. https://doi.org/10.1016/j.pneurobio.2007.04.002
Taiwo YO (1990) Levine JD (1990) Direct cutaneous hyperalgesia induced by adenosine. Neuroscience 38(3):757–762. https://doi.org/10.1016/0306-4522(90)90068-f
Taiwo YO, Levine JD (1991) Further confirmation of the role of adenyl cyclase and of cAMP-dependent protein kinase in primary afferent hyperalgesia. Neuroscience 44(1):131–135. https://doi.org/10.1016/0306-4522(91)90255-m
Khasar SG, Wang JF, Taiwo YO, Heller PH, Green PG, Levine JD (1995) Mu-opioid agonist enhancement of prostaglandin-induced hyperalgesia in the rat: a G-protein beta gamma subunit-mediated effect? Neuroscience 67(1):189–195. https://doi.org/10.1016/0306-4522(94)00632-f
Guntz E, Dumont H, Pastijn E, d’Exaerde Ade K, Azdad K, Sosnowski M, Schiffmann SN, Gall D (2008) Expression of adenosine A 2A receptors in the rat lumbar spinal cord and implications in the modulation of N-methyl-d-aspartate receptor currents. Anesth Analg 106(6):1882–1889. https://doi.org/10.1213/ane.0b013e318173251f
Kwilasz AJ, Ellis A, Wieseler J, Loram L, Favret J, McFadden A, Springer K, Falci S, Rieger J, Maier SF, Watkins LR (2018) Sustained reversal of central neuropathic pain induced by a single intrathecal injection of adenosine A2A receptor agonists. Brain Behav Immun 69:470–479. https://doi.org/10.1016/j.bbi.2018.01.005
Loram LC, Taylor FR, Strand KA, Harrison JA, Rzasalynn R, Sholar P, Rieger J, Maier SF, Watkins LR (2013) Intrathecal injection of adenosine 2A receptor agonists reversed neuropathic allodynia through protein kinase (PK)A/PKC signaling. Brain Behav Immun 33:112–122. https://doi.org/10.1016/j.bbi.2013.06.004
Coppi E, Cherchi F, Lucarini E, Ghelardini C, Pedata F, Jacobson KA, Di Cesare ML, Pugliese AM, Salvemini D (2021) Uncovering the mechanisms of adenosine receptor-mediated pain control: focus on the A3 receptor subtype. Int J Mol Sci 22(15):7952. https://doi.org/10.3390/ijms22157952
Hu X, Adebiyi MG, Luo J, Sun K, Le TT, Zhang Y, Wu H, Zhao S, Karmouty-Quintana H, Liu H, Huang A, Wen YE, Zaika OL, Mamenko M, Pochynyuk OM, Kellems RE, Eltzschig HK, Blackburn MR, Walters ET, Huang D, Hu H, Xia Y (2016) Sustained elevated adenosine via ADORA2B promotes chronic pain through neuro-immune interaction. Cell Rep 16(1):106–119. https://doi.org/10.1016/j.celrep.2016.05.080
Chen Z, Janes K, Chen C, Doyle T, Bryant L, Tosh DK, Jacobson KA, Salvemini D (2012) Controlling murine and rat chronic pain through A3 adenosine receptor activation. FASEB J 26(5):1855–1865. https://doi.org/10.1096/fj.11-201541
Zhang M, Hu H, Zhang X, Lu W, Lim J, Eysteinsson T, Jacobson KA, Laties AM, Mitchell CH (2010) The A3 adenosine receptor attenuates the calcium rise triggered by NMDA receptors in retinal ganglion cells. Neurochem Int 56(1):35–41. https://doi.org/10.1016/j.neuint.2009.08.011
Mintz IM, Sabatini BL, Regehr WG (1995) Calcium control of transmitter release at a cerebellar synapse. Neuron 15(3):675–688. https://doi.org/10.1016/0896-6273(95)90155-8
Hampson RE, Miller F, Palchik G, Deadwyler SA (2011) Cannabinoid receptor activation modifies NMDA receptor mediated release of intracellular calcium: implications for endocannabinoid control of hippocampal neural plasticity. Neuropharmacology 60(6):944–952. https://doi.org/10.1016/j.neuropharm.2011.01.039
Ford A, Castonguay A, Cottet M, Little JW, Chen Z, Symons-Liguori AM, Doyle T, Egan TM, Vanderah TW, De Koninck Y, Tosh DK, Jacobson KA, Salvemini D (2015) Engagement of the GABA to KCC2 signaling pathway contributes to the analgesic effects of A3AR agonists in neuropathic pain. J Neurosci 35(15):6057–6067. https://doi.org/10.1523/JNEUROSCI.4495-14.2015
Terayama R, Tabata M, Maruhama K, Iida S (2018) A3 adenosine receptor agonist attenuates neuropathic pain by suppressing activation of microglia and convergence of nociceptive inputs in the spinal dorsal horn. Exp Brain Res 236(12):3203–3213. https://doi.org/10.1007/s00221-018-5377-1
Wu WP, Hao JX, Halldner-Henriksson L, Xu XJ, Jacobson MA, Wiesenfeld-Hallin Z, Fredholm BB (2002) Decreased inflammatory pain due to reduced carrageenan-induced inflammation in mice lacking adenosine A3 receptors. Neuroscience 114(3):523–527. https://doi.org/10.1016/s0306-4522(02)00273-7
Smith NJ, Milligan G (2010) Allostery at G protein-coupled receptor homo- and heteromers: uncharted pharmacological landscapes. Pharmacol Rev 62(4):701–725. https://doi.org/10.1124/pr.110.002667
Carriba P, Navarro G, Ciruela F, Ferré S, Casadó V, Agnati L, Cortés A, Mallol J, Fuxe K, Canela EI, Lluís C, Franco R (2008) Detection of heteromerization of more than two proteins by sequential BRET-FRET. Nat Methods 5(8):727–733. https://doi.org/10.1038/nmeth.1229
Ferré S, Karcz-Kubicha M, Hope BT, Popoli P, Burgueño J, Gutiérrez MA, Casadó V, Fuxe K, Goldberg SR, Lluis C, Franco R, Ciruela F (2002) Synergistic interaction between adenosine A2A and glutamate mGlu5 receptors: implications for striatal neuronal function. Proc Natl Acad Sci U S A 99(18):11940–11945. https://doi.org/10.1073/pnas.172393799
Franco R, Cordomí A, Llinas Del Torrent C, Lillo A, Serrano-Marín J, Navarro G, Pardo L (2021) Structure and function of adenosine receptor heteromers. Cell Mol Life Sci 78(8):3957–3968. https://doi.org/10.1007/s00018-021-03761-6
Briddon SJ, Gandía J, Amaral OB, Ferré S, Lluís C, Franco R, Hill SJ, Ciruela F (2008) Plasma membrane diffusion of G protein-coupled receptor oligomers. Biochim Biophys Acta 1783(12):2262–2268. https://doi.org/10.1016/j.bbamcr.2008.07.006
May LT, Bridge LJ, Stoddart LA, Briddon SJ, Hill SJ (2011) Allosteric interactions across native adenosine-A3 receptor homodimers: quantification using single-cell ligand-binding kinetics. FASEB J 25(10):3465–3476. https://doi.org/10.1096/fj.11-186296
Canals M, Burgueño J, Marcellino D, Cabello N, Canela EI, Mallol J, Agnati L, Ferré S, Bouvier M, Fuxe K, Ciruela F, Lluis C, Franco R (2004) Homodimerization of adenosine A2A receptors: qualitative and quantitative assessment by fluorescence and bioluminescence energy transfer. J Neurochem 88(3):726–734. https://doi.org/10.1046/j.1471-4159.2003.02200.x
Vidi PA, Chen J, Irudayaraj JM, Watts VJ (2008) Adenosine A(2A) receptors assemble into higher-order oligomers at the plasma membrane. FEBS Lett 582(29):3985–3990. https://doi.org/10.1016/j.febslet.2008.09.062
Gracia E, Moreno E, Cortés A, Lluís C, Mallol J, McCormick PJ, Canela EI, Casadó V (2013) Homodimerization of adenosine A1 receptors in brain cortex explains the biphasic effects of caffeine. Neuropharmacology 71:56–69. https://doi.org/10.1016/j.neuropharm.2013.03.005
Ciruela F, Casadó V, Rodrigues RJ, Luján R, Burgueño J, Canals M, Borycz J, Rebola N, Goldberg SR, Mallol J, Cortés A, Canela EI, López-Giménez JF, Milligan G, Lluis C, Cunha RA, Ferré S, Franco R (2006) Presynaptic control of striatal glutamatergic neurotransmission by adenosine A1–A2A receptor heteromers. J Neurosci 26(7):2080–2087. https://doi.org/10.1523/JNEUROSCI.3574-05.2006
Hinz S, Navarro G, Borroto-Escuela D, Seibt BF, Ammon YC, de Filippo E, Danish A, Lacher SK, Červinková B, Rafehi M, Fuxe K, Schiedel AC, Franco R, Müller CE (2018) Adenosine A2A receptor ligand recognition and signaling is blocked by A2B receptors. Oncotarget 9(17):13593–13611. https://doi.org/10.18632/oncotarget.24423
Lillo A, Martínez-Pinilla E, Reyes-Resina I, Navarro G, Franco R (2020) Adenosine A2A and A3 receptors are able to interact with each other. A further piece in the puzzle of adenosine receptor-mediated signaling. Int J Mol Sci 21(14):5070. https://doi.org/10.3390/ijms21145070
Nascimento FP, Macedo-Júnior SJ, Lapa-Costa FR, Cezar-Dos-Santos F, Santos ARS (2021) Inosine as a tool to understand and treat central nervous system disorders: a neglected actor? Front Neurosci 15:703783. https://doi.org/10.3389/fnins.2021.703783
Lichty JA (1889) Relation of uric acid to migraine. JAMA 33(14):837–839
Haig A (1887) The relation of a certain form of headache to the excretion of uric acid. Med Chir Trans 70:355–369. https://doi.org/10.1177/095952878707000124
Schmidt AP, Böhmer AE, Soares FA, Posso IP, Machado SB, Mendes FF, Portela LV, Souza DO (2010) Changes in purines concentration in the cerebrospinal fluid of patients experiencing pain: a case–control study. Neurosci Lett 474(2):69–73. https://doi.org/10.1016/j.neulet.2010.02.067
Fais A, Cacace E, Corda M, Era B, Peri M, Utzeri S, Ruggiero V (2013) Purine metabolites in fibromyalgia syndrome. Clin Biochem 46(1–2):37–39. https://doi.org/10.1016/j.clinbiochem.2012.09.009
Haig A (1889) On uric acid and arterial tension. Br Med J 1(1467):288–291. https://doi.org/10.1136/bmj.1.1467.288
Yazar T, Yazar HO, Aygün A, Karabacak V, Altunkaynak Y, Kirbaş D (2021) Evaluation of serum uric levels in migraine. Neurol Sci 42(2):705–709. https://doi.org/10.1007/s10072-020-04598-w
Schulte G (2002) Adenosine receptor signaling and the activation of mitogen-activated protein kinases. Institutionen för fysiologi och farmakologi/Department of Physiology and Pharmacology. https://openarchive.ki.se/xmlui/bitstream/handle/10616/37706/thesis.pdf?sequence=1&isAllowed=y
Welihinda AA, Kaur M, Greene K, Zhai Y, Amento EP (2016) The adenosine metabolite inosine is a functional agonist of the adenosine A2A receptor with a unique signaling bias. Cell Signal 28(6):552–560. https://doi.org/10.1016/j.cellsig.2016.02.010
Fredholm BB, Irenius E, Kull B, Schulte G (2001) Comparison of the potency of adenosine as an agonist at human adenosine receptors expressed in Chinese hamster ovary cells. Biochem Pharmacol 61(4):443–448. https://doi.org/10.1016/s0006-2952(00)00570-0
Shen H, Chen GJ, Harvey BK, Bickford PC, Wang Y (2005) Inosine reduces ischemic brain injury in rats. Stroke 36(3):654–659. https://doi.org/10.1161/01.STR.0000155747.15679.04
Welihinda AA, Kaur M, Raveendran KS, Amento EP (2018) Enhancement of inosine-mediated A2AR signaling through positive allosteric modulation. Cell Signal 42:227–235. https://doi.org/10.1016/j.cellsig.2017.11.002
Prewitt RL, Sullivan JM (1980) Vasoactivity of inosine in the rat. Pharmacol Res Commun 12(3):205–213. https://doi.org/10.1016/s0031-6989(80)80003-8
Jones CE, Mayer LR, Smith EE, Hurst TW (1981) Relaxation of the isolated coronary artery by inosine: noninvolvement of the adenosine receptor. J Cardiovasc Pharmacol 3(3):612–621. https://doi.org/10.1097/00005344-198105000-00019
Van der Meer P, de Jong JW (1990) Inosine transiently decreases coronary flow but potentiates vasodilation by adenosine. Am J Physiol 259(3 Pt 2):H759-765. https://doi.org/10.1152/ajpheart.1990.259.3.H759
Nascimento FP, Macedo-Júnior SJ, Pamplona FA, Luiz-Cerutti M, Córdova MM, Constantino L, Tasca CI, Dutra RC, Calixto JB, Reid A, Sawynok J, Santos AR (2015) Adenosine A1 receptor-dependent antinociception induced by inosine in mice: pharmacological, genetic and biochemical aspects. Mol Neurobiol 51(3):1368–1378. https://doi.org/10.1007/s12035-014-8815-5
Macedo-Júnior SJ, Nascimento FP, Luiz-Cerutti M, Santos ARS (2021) The role of peripheral adenosine receptors in glutamate-induced pain nociceptive behavior. Purinergic Signal 17(2):303–312. https://doi.org/10.1007/s11302-021-09781-y
Cinalli AR, Guarracino JF, Fernandez V, Roquel LI, Losavio AS (2013) Inosine induces presynaptic inhibition of acetylcholine release by activation of A3 adenosine receptors at the mouse neuromuscular junction. Br J Pharmacol 169(8):1810–1823. https://doi.org/10.1111/bph.12262
Isaacs H, Heffron JJ, Berman L, Badenhorst M, Pickering A (1975) Xanthine, hypoxanthine and muscle pain. Histochemical and biochemical observations. S Afr Med J 49(26):1035–1038
Goldstein KM (2017) Xanthine Oxidase Inhibitors and Their Role in Neuropathic Pain. Dissertation, Harvard University. http://nrs.harvard.edu/urn-3:HUL.InstRepos:37736766
Singh N, Shreshtha AK, Thakur MS, Patra S (2018) Xanthine scaffold: scope and potential in drug development. Heliyon 4(10):e00829. https://doi.org/10.1016/j.heliyon.2018.e00829
Bruns RF, Daly JW, Snyder SH (1983) Adenosine receptor binding: structure–activity analysis generates extremely potent xanthine antagonists. Proc Natl Acad Sci U S A 80(7):2077–2080. https://doi.org/10.1073/pnas.80.7.2077
Sautin YY, Johnson RJ (2008) Uric acid: the oxidant-antioxidant paradox. Nucleosides Nucleotides Nucleic Acids 27(6):608–619. https://doi.org/10.1080/15257770802138558
Waring WS (2002) Uric acid: an important antioxidant in acute ischaemic stroke. QJM 95(10):691–693. https://doi.org/10.1093/qjmed/95.10.691
Khosravi A, Nakhaee A, Ghoreishi A, Arefpoor Z, Sadeghi M (2019) Impaired oxidative-antioxidative balance during migraine attack. Biomed Res Ther 6(2):2996–3002
Togha M, RazeghiJahromi S, Ghorbani Z, Ghaemi A, Rafiee P (2019) An investigation of oxidant/antioxidant balance in patients with migraine: a case–control study. BMC Neurol 19(1):323. https://doi.org/10.1186/s12883-019-1555-4
Sodani K, Patel A, Kathawala RJ, Chen ZS (2012) Multidrug resistance associated proteins in multidrug resistance. Chin J Cancer 31(2):58–72. https://doi.org/10.5732/cjc.011.10329
Anthony M (1981) Biochemical indices of sympathetic activity in migraine. Cephalalgia 1(2):83–89. https://doi.org/10.1111/j.1468-2982.1981.tb00014.x
Birk S, Kruuse C, Petersen KA, Tfelt-Hansen P, Olesen J (2006) The headache-inducing effect of cilostazol in human volunteers. Cephalalgia 26(11):1304–1309. https://doi.org/10.1111/j.1468-2982.2006.01218.x
Guo S, Olesen J (2014) Ashina M (2014) Phosphodiesterase 3 inhibitor cilostazol induces migraine-like attacks via cyclic AMP increase. Brain 137(Pt 11):2951–2959. https://doi.org/10.1093/brain/awu244
Younis S, Christensen CE, Toft NM, Søborg T, Amin FM, Hougaard A, Ashina M (2019) Investigation of distinct molecular pathways in migraine induction using calcitonin gene-related peptide and sildenafil. Cephalalgia 39(14):1776–1788. https://doi.org/10.1177/0333102419882474
Galeotti N, Ghelardini C, Zoppi M, Del Bene E, Raimondi L, Beneforti E, Bartolini A (2001) Hypofunctionality of Gi proteins as aetiopathogenic mechanism for migraine and cluster headache. Cephalalgia 21(1):38–45. https://doi.org/10.1046/j.1468-2982.2001.00142.x
Lincoln TM, Cornwell TL (1991) Towards an understanding of the mechanism of action of cyclic AMP and cyclic GMP in smooth muscle relaxation. Blood Vessels 28(1–3):129–137. https://doi.org/10.1159/000158852
White RE, Kryman JP, El-Mowafy AM, Han G, Carrier GO (2000) cAMP-dependent vasodilators cross-activate the cGMP-dependent protein kinase to stimulate BK(Ca) channel activity in coronary artery smooth muscle cells. Circ Res 86(8):897–905. https://doi.org/10.1161/01.res.86.8.897
Kokoti L, Al-Karagholi MA, Ashina M (2020) Latest insights into the pathophysiology of migraine: the ATP-sensitive potassium channels. Curr Pain Headache Rep 24(12):77. https://doi.org/10.1007/s11916-020-00911-6
Thuraiaiyah J, Kokoti L, Al-Karagholi MA, Ashina M (2022) Involvement of adenosine signaling pathway in migraine pathophysiology: a systematic review of preclinical studies. J Headache Pain 23(1):43. https://doi.org/10.1186/s10194-022-01412-0
Cieślak M, Czarnecka J, Roszek K, Komoszyński M (2015) The role of purinergic signaling in the etiology of migraine and novel antimigraine treatment. Purinergic Signal 11(3):307–316. https://doi.org/10.1007/s11302-015-9453-8
Haanes KA, Edvinsson L (2019) Pathophysiological mechanisms in migraine and the identification of new therapeutic targets. CNS Drugs 33(6):525–537. https://doi.org/10.1007/s40263-019-00630-6
Nowaczewska M, Wiciński M, Kaźmierczak W (2020) The ambiguous role of caffeine in migraine headache: from trigger to treatment. Nutrients 12(8):2259. https://doi.org/10.3390/nu12082259
Dorsam RT, Murugappan S, Ding Z, Kunapuli SP (2003) Clopidogrel: interactions with the P2Y12 receptor and clinical relevance. Hematology 8(6):359–365. https://doi.org/10.1080/10245330310001621260
Chambers JB, Seed PT, Ridsdale L (2014) Clopidogrel as prophylactic treatment for migraine: a pilot randomised, controlled study. Cephalalgia 34(14):1163–1168. https://doi.org/10.1177/0333102414531156
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by RGB. The first draft of the manuscript was written by RGB, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Biringer, R.G. Migraine signaling pathways: purine metabolites that regulate migraine and predispose migraineurs to headache. Mol Cell Biochem 478, 2813–2848 (2023). https://doi.org/10.1007/s11010-023-04701-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-023-04701-7