Abstract
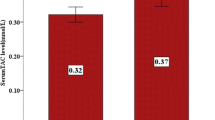
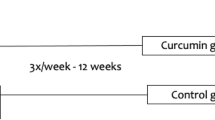
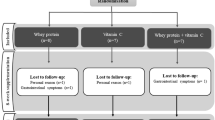
The aim of our study was to investigate the effects of one-month consumption of polyphenol-rich standardized Aronia melanocarpa extract (SAE) on redox status in anemic hemodialysis patients. The study included 30 patients (Hb < 110 g/l, hemodialysis or hemodiafiltration > 3 months; > 3 times week). Patients were treated with commercially available SAE in a dose of 30 ml/day, for 30 days. After finishing the treatment blood samples were taken to evaluate the effects of SAE on redox status. Several parameters of anemia and inflammation were also followed. After the completion of the treatment, the levels of superoxide anion radical and nitrites significantly dropped, while the antioxidant capacity improved via elevation of catalase and reduced glutathione. Proven antioxidant effect was followed by beneficial effects on anemia parameters (increased hemoglobin and haptoglobin concentration, decreased ferritin and lactate dehydrogenase concentration), but SAE consumption didn't improve inflammatory status, except for minor decrease in C-reactive protein. The consumption of SAE regulates redox status (reduce the productions of pro-oxidative molecules and increase antioxidant defense) and has beneficial effects on anemia parameters. SAE could be considered as supportive therapy in patients receiving hemodialysis which are prone to oxidative stress caused by both chronic kidney disease and hemodialysis procedure. Additionally, it could potentially be a good choice for supplementation of anemic hemodialysis patients. TRN: NCT04208451 December 23, 2019 “retrospectively registered”



Similar content being viewed by others
Data availability
Data are available from the correspondence author upon reasonable request.
References
Pisoni RL, Bragg-Gresham JL, Young EW, Akizawa T, Asano Y, Locatelli F, Bommer J, Cruz JM, Kerr PG, Mendelssohn DC, Held PJ, Port FK (2004) Anemia management and outcomes from 12 countries in the dialysis outcomes and practice patterns study (DOPPS). Am J Kidney Dis 44:94–111. https://doi.org/10.1053/j.ajkd.2004.03.023
Locatelli F, Pisoni RL, Combe C, Bommer J, Andreucci VE, Piera L, Greenwood R, Feldman HI, Port FK, Held PJ (2004) Anaemia in haemodialysis patients of five European countries: association with morbidity and mortality in the dialysis outcomes and practice patterns study (DOPPS). Nephrol Dial Transplant 19:121–132. https://doi.org/10.1093/ndt/gfg458
Babitt JL, Lin HY (2012) Mechanisms of anemia in CKD. J Am Soc Nephrol 23:1631–1634. https://doi.org/10.1681/ASN.2011111078
Vaziri ND (2004) Roles of oxidative stress and antioxidant therapy in chronic kidney disease and hypertension. Curr Opin Nephrol Hypertens 13:93–99. https://doi.org/10.1097/00041552-200401000-00013
Bayes B, Pastor MC, Bonal J, Juncà J, Romero R (2001) Homocysteine and lipid peroxidation in haemodialysis: role of folinic acid and vitamin E. Nephrol Dial Transplant 16:2172–2175. https://doi.org/10.1093/ndt/16.11.2172
Morena M, Cristol JP, Bosc JY, Tetta C, Forret G, Leger CL, Delcourt C, Papoz L, Descomps B, Canaud B (2002) Convective and diffusive losses of vitamin C during haemodiafiltration session: a contributive factor to oxidative stress in haemodialysis patients. Nephrol Dial Transplant 17:422–427. https://doi.org/10.1093/ndt/17.3.422
Spittle MA, Hoenich NA, Handelman GJ, Adhikarla R, Homel P, Levin NW (2001) Oxidative stress and inflammation in hemodialysis patients. Am J Kidney Dis 38:1408–1413. https://doi.org/10.1053/ajkd.2001.29280
Oberg BP, McMenamin E, Lucas FL, McMonagle E, Morrow J, Ikizler TA, Himmelfarb J (2004) Increased prevalence of oxidant stress and inflammation in patients with moderate to severe chronic kidney disease. Kidney Int 65:1009–1016. https://doi.org/10.1111/j.1523-1755.2004.00465.x
Simmons EM, Langone A, Sezer MT, Vella JP, Recupero P, Morrow JD, Ikizler TA, Himmelfarb J (2005) Effect of renal transplantation on biomarkers of inflammation and oxidative stress in end-stage renal disease patients. Transplantation 79:914–919. https://doi.org/10.1097/01.TP.0000157773.96534.29
Hill NR, Fatoba ST, Oke JL, Hirst JA, O’Callaghan CA, Lasserson DS, Hobbs FD (2016) Global prevalence of chronic kidney disease—a systematic review and meta-analysis. PLoS One 11:e0158765. https://doi.org/10.1371/journal.pone.0158765
Fishbane S, Spinowitz B (2017) Update on anemia in ESRD and earlier stages of CKD: core curriculum. Am J Kidney Dis 71:423–435. https://doi.org/10.1053/j.ajkd.2017.09.026
Himmelfarb J, Phinney S, Ikizler TA, Kane J, Mconagle E, Miller G (2007) Gamma- tocopherol and docosahexaenoic acid decrease inflammation in dialysis patients. J Ren Nutr 17:296–304. https://doi.org/10.1053/j.jrn.2007.05.011
Roger SD (2017) Practical considerations for iron therapy in the management of anemia in patients with chronic kidney disease. Clin Kidney J. https://doi.org/10.1093/ckj/sfx100
Kamgar M, Zaldivar F, Vaziri ND, Pahl MV (2009) Antioxidant therapy does not ameliorate oxidative stress and inflammation in patients with end-stage renal disease. J Natl Med Assoc 101:336–344. https://doi.org/10.1016/S0027-9684(15)30881-6
Shema-Didi L, Kristal B, Ore L, Shapiro G, Geron R, Sela S (2013) Pomegranate juice intake attenuates the increase in oxidative stress induced by intravenous iron during heamodialysis. Nutr Res 33:442–446. https://doi.org/10.1016/j.nutres.2013.04.004
Pakfetrat M, Akmali M, Malekmakan L, Dabaghimanesh M, Khorsand M (2015) Role of turmeric in oxidative modulation in end-stage renal disease patients. Hemodial Int 19:124–131. https://doi.org/10.1111/hdi.12204
Rassaf T, Rammos C, Hendgen-Cotta UB, Heiss C, Kleophas W, Dellanna F, Floege J, Hetzel GR, Kelm M (2016) Vasculoprotective effects of dietary cocoa flavanols in patients on haemodialysis: a double-blind, randomized, placebo-controlled trial. Clin J Am Soc Nephrol 11:108–118. https://doi.org/10.2215/CJN.05560515
Janiques AG, de Oliviera LV, Stockler-Pinto MB, Moreira NX, Mafra D (2014) Effects of grape powder supplementation on inflammatory and antioxidant markers in haemodialysis patients: a randomized double-blind study. J Bras Nefrol 36:496–501. https://doi.org/10.5935/0101-2800.20140071
Shema-Didi L, Sela S, Ore L (2012) One year of pomegranate juice intake decreases oxidative stress, inflammation, and incidence of infections in haemodialysis patients: a randomized placebo-controlled trial. Free Radic Biol Med 53:297–304. https://doi.org/10.1016/j.freeradbiomed.2012.05.013
Pakfetrat M, Basiri F, Malekmakan L, Roozbeh J (2014) Effects of turmeric on uremic pruritus in end stage renal disease patients: A double-blind randomized clinical trial. J Nephrol 27:203–207. https://doi.org/10.1007/s40620-014-0039-2
Daskalova E, Delchev S, Peeva Y, Vladimirova-Kitova L, Kratchanova M, Kratchanov C, Denev P (2015) Antiatherogenic and cardioprotective effects of black chokeberry (Aronia melanocarpa) juice in aging rats. Evid Based Complement Alternat Med 2015:717439. https://doi.org/10.1155/2015/717439
Kokotkiewicz A, Jaremicz Z, Luczkiewicz M (2010) Aronia plants: a review of traditional use, biological activities, and perspectives for modern medicine. J Med Food 13:255–269. https://doi.org/10.1089/jmf.2009.0062
Ochmian I, Grajkowski J, Smolik M (2012) Comparison of some morphological features, quality and chemical content of four cultivars of chokeberry fruits (Aronia melanocarpa). Not Bot Horti Agrobot Cluj Napoca 40:253–60. https://doi.org/10.15835/nbha4017181
Cebova M, Klimentova J, Janega P, Pechanova O (2017) Effect of bioactive compound of Aronia melanocarpa on cardiovascular system in experimental hypertension. Oxid Med Cell Longev 2017:8156594. https://doi.org/10.1155/2017/8156594
Jankowski A, Jankowska B, Niedworok J (2000) The influence of Aronia melanocapra in experimental pancreatitis. Pol Merkur Lekarski 8(48):395–398
Minato K, Miyake Y, Fukumoto S, Yamamoto K, Kato Y, Shimomura Y, Osawa T (2003) Lemon flavonoid, eriocitrin, suppresses exercise-induced oxidative damage in rat liver. Life Sci 72(14):1609–1616. https://doi.org/10.1016/s0024-3205(02)02443-8
Jurikova T, Mlcek J, Skrovankova S, Sumczynski D, Sochor J, Hlavacova I, Snopek L, Orsavova J (2017) Fruits of black chokeberry Aronia melanocarpa in the prevention of chronic diseases. Molecules 22:944. https://doi.org/10.3390/molecules22060944
Banjari I, Misir A, Šavikin K, Molnar M, De Zoysa HKS, Waisundara VY (2017) Antidiabetic effects of Aronia melanocarpa and its other therapeutic properties. Front Nutr 4:53. https://doi.org/10.3389/fnut.2017.00053
Broncel M, Kozirog M, Duchnowicz P, Koter-Michalak M, Sikora J, Chojnowska-Jezierska J (2010) Aronia melanocarpa extract reduces blood pressure, serum endothelin, lipid, and oxidative stress marker levels in patients with metabolic syndrome. Med Sci Monit 16:CR28-34
Jakovljevic V, Milic P, Bradic J, Jeremic J, Zivkovic V, Srejovic I, Nikolic Turnic T, Milosavljevic I, Jeremic N, Bolevich S, Labudovic Borovic M, Mitrovic M, Vucic V (2018) Standardized Aronia melanocarpa extract as novel supplement against metabolic syndrome: a rat model. Int J Mol Sci 20:6. https://doi.org/10.3390/ijms20010006
Milic P, Jeremic J, Zivkovic V, Srejovic I, Jeremic N, Bradic J, Nikolic Turinic T, Milosavljevic I, Bolevich S, Bolevich S, Labudovic Borovic M, Arsic A, Mitrovic M, Jakovljevic V, Vucic V (2019) Effects of different dietary regimes alone or in combination with standardized Aronia melanocarpa extract supplementation on lipid and fatty acids profiles in rats. Mol Cell Biochem 461(1–2):141–150
Antic S, Draginic N, Pilcevic D, Zivkovic V, Srejovic I, Jeremic N, Petrovic D, Jakovljevic V (2021) The influence of vitamin E coated dialysis membrane on oxidative stress during the single session of on-line hemodiafiltration. Vojnosanit Pregl 00:97–97. https://doi.org/10.2298/VSP190730097A
Mattera R, Benvenuto M, Giganti M, Tresoldi I, Pluchinotta FR, Bergante S, Tettamanti G, Masuelli L, Manzari V, Modesti A, Bei R (2017) Effects of polyphenols on oxidative stress-mediated injury in cardiomyocytes. Nutrients 9:523. https://doi.org/10.3390/nu9050523
Mojzer EB, Hrncic MK, Skerget M, Knez Z, Bren U (2016) Polyphenols: extraction methods, antioxidative action, bioavailability and anticarcinogenic effects. Molecules 21:901. https://doi.org/10.3390/molecules21070901
McMahon M, Itoh K, Yamamoto M, Hayes JD (2003) Keap1-dependent proteasomal degradation of transcription factor Nrf2 contributes to the negative regulation of antioxidant response element-driven gene expression. J Biol Chem 278(24):21592–21600. https://doi.org/10.1074/jbc.M300931200
Pergola C, Rossi A, Dugo P, Cuzzocrea S, Sautebin L (2006) Inhibition of nitric oxide biosynthesis by anthocyanin fraction of blackberry extract. Nitric Oxide 15(1):30–39. https://doi.org/10.1016/j.niox.2005.10.003
Xu JW, Ikeda K, Yamori Y (2004) Upregulation of endothelial nitric oxide synthase by cyanidin-3-glucoside, a typical anthocyanin pigment. Hypertension 44:217–222
Taheri R, Connolly BA, Brand MH, Bolling BW (2013) Underutilized chokeberry (Aroniamelanocarpa, Aroniaarbutifolia, Aroniaprunifolia) accessions are rich sources of anthocyanins, flavonoids, hydroxycinnamic acids, and proanthocyanidins. J Agric Food Chem 61:8581–8588. https://doi.org/10.1021/jf402449q
Khor BH, Narayanan SS, Sahathevan S, Gafor AHA, Daud ZAM, Khosla P, Sabatino A, Fiaccadori E, Chinna K, Karupaiah T (2018) Efficacy of nutritional interventions on inflammatory markers in haemodialysis patients: a systematic review and limited meta-analysis. Nutrients 10:397. https://doi.org/10.3390/nu10040397
Kang SH, Jeon YD, Moon KH, Lee KH, Kim DG, Kima W, Myung H, Kim JS, Kim HJ, Bang KS, Jin JS (2017) Aronia berry extract ameliorates the severity of dextran sodium sulfate-induced ulcerative colitis in mice. J Med Food 20:667–675. https://doi.org/10.1089/jmf.2016.3822
Spormann TM, Albert FW, Rath T, Dietrich T, Will F, Stockis JP, Eisenbrand G, Janzowski C (2008) Anthocyanin/polyphenolic-rich fruit juice reduces oxidative cell damage in an intervention study with patients on heamodialysis. Cancer Epidemiol Biomarkers Prev 17:3373–3380. https://doi.org/10.1158/1055-9965.EPI-08-0364
Santos EJF, Hortegal EV, Serra HO, Lages JS, Salgado-Finho N, Dos Santos AA (2018) Epoetin alfa resistance in hemodialysis patients with chronic kidney disease: a longitudinal study. Braz J Med Biol Res 51:e7288. https://doi.org/10.1590/1414-431x20187288
Shih AWY, Mcfarlane A, Verhovsek M (2014) Haptoglobin testing in hemolysis: measurement and interpretation. Am J Hematol 89:443–447. https://doi.org/10.1002/ajh.23623
Barcellini W, Fattizzo B, Zaninoni A, Radice T, Nichele I, Di Bona E, Lunghi M, Tassinari C, Alfinito F, Ferrari A, Leporace AP, Niscola P, Carpenedo M, Boschetti C, Revelli N, Villa MA, Consonni D, Scaramucci L, De Fabritiis P, Tagariello G, Gaidano G, Rodeghiero F, Cortelezzi A, Zanella A (2014) Clinical heterogeneity and predictors of outcome in primary autoimmune hemolytic anemia: a GIMEMA study of 308 patients. Blood 124:2930–2936. https://doi.org/10.1182/blood-2014-06-583021
Acknowledgements
This research was supported by the Faculty of Medical Sciences, University of Kragujevac. Authors express special thanks to Pharmanova, Belgrade, Serbia for donation of polyphenol-rich standardized Aronia melanocarpa extract.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
Conceptualization, V.J. and S.B.; Writing–original draft preparation, I.M. and N.D.; Methodology, D.P. and N.A.; Software, V.S.; Resources, M.M.; Investigation, J.J. and I.S.; Supervision, V.Z. All authors contributed substantially to the work reported. All authors have read and agreed to the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
Study is approved by the Ethics Committee of the Clinical Center Kragujevac, Serbia No 01-14-3039.
Consent to participate
All patients gave written informed consent to participate in the study.
Consent for publication
All patients gave written informed consent for publication of data.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Milosavljevic, I., Jakovljevic, V., Petrovic, D. et al. Standardized Aronia melanocarpa extract regulates redox status in patients receiving hemodialysis with anemia. Mol Cell Biochem 476, 4167–4175 (2021). https://doi.org/10.1007/s11010-021-04225-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-021-04225-y