Abstract
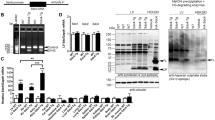
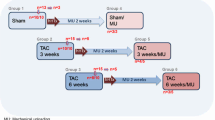
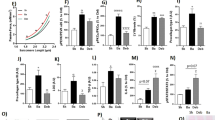
Transition from compensated to decompensated left ventricular hypertrophy (LVH) is accompanied by functional and structural changes. Here, the aim was to evaluate dystrophin expression in murine models and human subjects with LVH by transverse aortic constriction (TAC) and aortic stenosis (AS), respectively. We determined whether doxycycline (Doxy) prevented dystrophin expression and myocardial stiffness in mice. Additionally, ventricular function recovery was evaluated in patients 1 year after surgery. Mice were subjected to TAC and monitored for 3 weeks. A second group received Doxy treatment after TAC. Patients with AS were stratified by normal left ventricular end-diastolic wall stress (LVEDWS) and high LVEDWS, and groups were compared. In mice, LVH decreased inotropism and increased myocardial stiffness associated with a dystrophin breakdown and a decreased mitochondrial O2 uptake (MitoMVO2). These alterations were attenuated by Doxy. Patients with high LVEDWS showed similar results to those observed in mice. A correlation between dystrophin and myocardial stiffness was observed in both mice and humans. Systolic function at 1 year post-surgery was only recovered in the normal-LVEDWS group. In summary, mice and humans present diastolic dysfunction associated with dystrophin degradation. The recovery of ventricular function was observed only in patients with normal LVEDWS and without dystrophin degradation. In mice, Doxy improved MitoMVO2. Based on our results it is concluded that the LVH with high LVEDWS is associated to a degradation of dystrophin and increase of myocardial stiffness. At least in a murine model these alterations were attenuated after the administration of a matrix metalloprotease inhibitor.





Similar content being viewed by others
References
Hein S, Kostin S, Heling A, Maeno Y, Schaper J (2000) The role of the cytoskeleton in heart failure. Cardiovasc Res 45:273–278. doi:10.1016/S0008-6363(99)00268-0
Toyo-Oka T, Kawada T, Nakata J, Xie H, Urabe M, Masui F, Ebisawa T, Tezuka A, Iwasawa K, Nakajima T, Uehara Y, Kumagai H, Kostin S, Schaper J, Nakazawa M, Ozawa K (2004) Translocation and cleavage of myocardial dystrophin as a common pathway to advanced heart failure: a scheme for the progression of cardiac dysfunction. Proc Natl Acad Sci USA 101:7381–7385. doi:10.1073/pnas.0401944101
Kawada T, Masui F, Tezuka A, Ebisawa T, Kumagai H, Nakazawa M, Toyo-Oka T (2005) A novel scheme of dystrophin disruption for the progression of advanced heart failure. Biochim Biophys 1751:73–81. doi:10.1016/j.bbapap.2005.01.001
Townsend D, Turner I, Yasuda S, Martindale J, Davis J, Shillingford M, Kornegay JN, Metzger JM (2010) Chronic administration of membrane sealant prevents severe cardiac injury and ventricular dilatation in dystrophic dogs. J Clin Investig 120:1140–1150. doi:10.1172/JCI41329
Townsend D, Yasuda S, McNally E, Metzger JM (2011) Distinct pathophysiological mechanisms of cardiomyopathy in hearts lacking dystrophin or the sarcoglycan complex. FASEB J 25:3106–3114. doi:10.1096/fj.10-178913
Han F, Lu YM, Hasegawa H, Kanai H, Hachimura E, Shirasaki Y, Fukunaga K (2010) Inhibition of dystrophin breakdown and endothelial nitric-oxide synthase uncoupling accounts for cytoprotection by 3-[2-[4-(3-chloro-2-methylphenyl)-1-piperazinyl]ethyl]-5,6-dimethoxy-1-(4-imidazolylmethyl)-1H-indazole dihydrochloride 3.5 hydrate (DY-9760e) in left ventricular hypertrophied mice. J Pharmacol Exp Ther 332:421–428. doi:10.1124/jpet.109.161646
Buchholz B, Perez V, Siachoque N, Miksztowicz V, Berg G, Rodríguez M, Donato M, Gelpi RJ (2014) Dystrophin proteolysis: a potential target for MMP-2 and its prevention by ischemic preconditioning. Am J Physiol Heart Circ Physiol 307:88–96. doi:10.1152/ajpheart.00242.2013
Yarbrough WM, Mukherjee R, Ikonomidis JS, Zile MR, Spinale FG (2012) Myocardial remodeling with aortic stenosis and after aortic valve replacement: mechanisms and future prognostic implications. J Thorac Cardiovasc Surg 143:656–664. doi:10.1016/j.jtcvs.2011.04.044
Sahn DJ, DeMaria A, Kisslo J, Weyman A (1978) Recommendations regarding quantitation in M-mode echocardiography: results of a survey of echocardiographic measurements. Circulation 58:1072–1083. doi:10.1161/01.CIR.58.6.1072
Errami M, Galindo CL, Tassa AT, Dimaio JM, Hill JA, Garner HR (2008) Doxycycline attenuates isoproterenol and transverse aortic banding-induced cardiac hypertrophy in mice. J Pharmacol Exp Ther 324:1196–1203. doi:10.1124/jpet.107.133975
Villarreal FJ, Griffin M, Omens J, Dillmann W, Nguyen J, Covell J (2003) Early short-term treatment with doxycycline modulates postinfarction left ventricular remodeling. Circulation 108:1487–1492. doi:10.1161/01.CIR.0000089090.05757.34
Wang G, Bergman M, Nguyen A, Turcato S, Swigart P, Rodrigo M, Simpson PC, Karliner JS, Lovett DH, Baker AJ (2006) Cardiac transgenic matrix metalloproteinase-2 expression directly induces impaired contractility. Cardiovasc Res 69:688–696. doi:10.1016/j.cardiores.2005.08.023
Rork TH, Hadzimichalis NM, Kappil MA, Merrill GF (2006) Acetaminophen attenuates peroxynitrite-activated matrix metalloproteinase-2-mediated troponin I cleavage in the isolated guinea pig myocardium. J Mol Cell Cardiol 40:553–561. doi:10.1016/j.yjmcc.2006.01.010
Sawicki G, Leon H, Sawicka J, Sariahmetoglu M, Schulze CJ, Scott PG, Szczesna-Cordary D, Schulz R (2005) Degradation of myosin light chain in isolated rat hearts subjected to ischemia-reperfusion injury: a new intracellular target for matrix metalloproteinase-2. Circulation 112:544–552. doi:10.1161/CIRCULATIONAHA.104.531616
Jalil JE, Doering CW, Janicki JS, Pick R, Shroff SG, Weber KT (1989) Fibrillar collagen and myocardial stiffness in the intact hypertrophied rat left ventricle. Circ Res 64:1041–1050. doi:10.1161/01.RES.64.6.1041
Matsusaka H, Ide T, Matsushima S, Ikeuchi M, Kubota T, Sunagawa K, Kinugawa S, Tsutsui H (2006) Targeted deletion of matrix metalloproteinase 2 ameliorates myocardial remodeling in mice with chronic pressure overload. Hypertension 47:711–717. doi:10.1161/01.HYP.0000208840.30778.00
Conrad CH, Brooks WW, Hayes JA, Sen S, Robinson KG, Bing OH (1995) Myocardial fibrosis and stiffness with hypertrophy and heart failure in the spontaneously hypertensive rat. Circulation 91:161–170. doi:10.1161/01.CIR.91.1.161
Gelpi RJ, Gao S, Zhai P, Yan L, Hong C, Danridge LM, Ge H, Maejima Y, Donato M, Yokota M, Molkentin JD, Vatner DE, Vatner SF, Sadoshima J (2009) Genetic inhibition of calcineurin induces diastolic dysfunction in mice with chronic pressure overload. Am J Physiol Heart Circ Physiol 297:1814–1819. doi:10.1152/ajpheart.00449.2009
Stuyvers BD, Miura M, ter Keurs HE (2000) Ca (2+) dependence of passive properties of cardiac sarcomeres. Adv Exp Med Biol 481:353–366
Akhmedov AT, Rybin V, Marín-García J (2015) Mitochondrial oxidative metabolism and uncoupling proteins in the failing heart. Heart Fail Rev 20:227–249. doi:10.1007/s10741-014-9457-4
Zhou LY, Liu JP, Wang K, Gao J, Ding SL, Jiao JQ, Li PF (2013) Mitochondrial function in cardiac hypertrophy. Int J Cardiol 167:1118–1125. doi:10.1016/j.ijcard.2012.09.082
Cheung PY, Sawicki G, Wozniak M, Wang W, Radomski MW, Schulz R (2000) Matrix metalloproteinase-2 contributes to ischemia-reperfusion injury in the heart. Circulation 101:1833–1839 doi:10.1161/01.CIR.101.15.1833
Acknowledgements
We thank Dr. Liliana Grinfeld for assistance in recruiting patients in the control group and for additional invaluable support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
none declared.
Additional information
Martín Donato, Bruno Buchholz, Laura Valdez, Tamara Zaobornyj, Alberto Boveris, and Ricardo J. Gelpi are members of the National Council of Scientific and Technological Research (CONICET).
Rights and permissions
About this article
Cite this article
Donato, M., Buchholz, B., Morales, C. et al. Loss of dystrophin is associated with increased myocardial stiffness in a model of left ventricular hypertrophy. Mol Cell Biochem 432, 169–178 (2017). https://doi.org/10.1007/s11010-017-3007-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-017-3007-z