Abstract
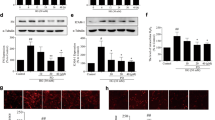
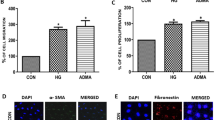
Both NADPH oxidase (NOX) and inducible nitric oxide synthase (iNOS) are the main sources of reactive oxygen species in kidney. However, their interactions in oxidative stress and contributions to kidney fibrosis during diabetic nephropathy have not been studied. Human mesangial cells were treated with normal glucose (5.6 mmol/L), high glucose (30 mmol/L) in the presence or absence of AGE (200 mg/L). Protein expressions of NOX1, NOX2, NOX4, and iNOS were examined by immunoblotting. NOX was genetically silenced with specific RNAi to study the interactions between NOX and iNOS in diabetic milieu. Superoxide (O·−) and peroxynitrite (ONOO·−) productions were assessed by dihydroethidium and hydroxyphenyl fluorescein, respectively. Fibrotic factors were determined by biochemistry assay. Superoxide, peroxynitrite, TGF-β, and fibronectin productions as well as the protein expressions of NOX1, NOX2, NOX4, and iNOS were increased in the diabetic milieu (high glucose 30 mmol/L plus AGE 200 mg/L). However, abolishment of iNOS induction with 1400W or iNOS RNAi would restore peroxynitrite, TGF-β, and fibronectin productions completely to basal level and attenuate superoxide production. Moreover, NOX1 inhibition not only prevented iNOS induction but also abrogated changes consequent to iNOS induction such as mesangial fibrogenesis.





Similar content being viewed by others
References
Lopes AA (2009) End-stage renal disease due to diabetes in racial/ethnic minorities and disadvantaged populations. Ethn Dis 19(1):47
Palm F, Nordquist L, Wilcox CS, Hansell P (2011) Oxidative stress and hypoxia in the pathogenesis of diabetic nephropathy. Studies on renal disorders. Humana Press, NY, pp 559–586
Trachtman H, Futterweit S, Pine E, Mann J, Valderrama E (2002) Chronic diabetic nephropathy: role of inducible nitric oxide synthase. Pediatr Nephrol 17(1):20–29. doi:10.1007/s004670200004
Piao YJ, Seo YH, Hong F, Kim JH, Kim YJ, Kang MH, Kim BS, Jo SA, Jo I, Jue DM, Kang I, Ha J, Kim SS (2005) Nox 2 stimulates muscle differentiation via NF-kappaB/iNOS pathway. Free Radic Biol Med 38(8):989–1001. doi:10.1016/j.freeradbiomed.2004.11.011
Fujii J (2011) Introduction to serial reviews: physiological relevance of antioxid/redox genes; learning from genetically modified animals. J Clin Biochem Nutr 49(2):69
Szabo C (2009) Role of nitrosative stress in the pathogenesis of diabetic vascular dysfunction. Br J Pharmacol 156(5):713–727
Thallas-Bonke V, Thorpe SR, Coughlan MT, Fukami K, Yap FYT, Sourris KC, Penfold SA, Bach LA, Cooper ME, Forbes JM (2008) Inhibition of NADPH oxidase prevents advanced glycation end product-mediated damage in diabetic nephropathy through a protein kinase C-α-dependent pathway. Diabetes 57(2):460–469
Huang J, Huang K, Lan T, Xie X, Shen X, Liu P, Huang H (2013) Curcumin ameliorates diabetic nephropathy by inhibiting the activation of the SphK1-S1P signaling pathway. Mol Cell Endocrinol 365(2):231–240. doi:10.1016/j.mce.2012.10.024
Xie X, Peng J, Chang X, Huang K, Huang J, Wang S, Shen X, Liu P, Huang H (2013) Activation of RhoA/ROCK regulates NF-kappaB signaling pathway in experimental diabetic nephropathy. Mol Cell Endocrinol 369(1–2):86–97. doi:10.1016/j.mce.2013.01.007
Xiao H, Li Y, Qi J, Wang H, Liu K (2009) Peroxynitrite plays a key role in glomerular lesions in diabetic rats. J Nephrol 22(6):800–808
Cui W, Li B, Bai Y, Miao X, Chen Q, Sun W, Tan Y, Luo P, Zhang C, Zheng S, Epstein PN, Miao L, Cai L (2013) Potential role for Nrf2 activation in the therapeutic effect of MG132 on diabetic nephropathy in OVE26 diabetic mice. Am J Physiol Endocrinol Metab 304(1):E87–E99. doi:10.1152/ajpendo.00430.2012
Star RA (1997) Intrarenal localization of nitric oxide synthase isoforms and soluble guanylyl cyclase. Clin Exp Pharmacol Physiol 24(8):607–610
Pawluczyk IZA, Harris KPG (2012) Effect of angiotensin type 2 receptor over-expression on the rat mesangial cell fibrotic phenotype: effect of gender. J Renin Angiotensin Aldosterone Syst 13(2):221–231
Chang PC, Chen TH, Chang CJ, Hou CC, Chan P, Lee HM (2004) Advanced glycosylation end products induce inducible nitric oxide synthase (iNOS) expression via a p38 MAPK-dependent pathway. Kidney Int 65(5):1664–1675. doi:10.1111/j.1523-1755.2004.00602.x
Sugimoto H, Shikata K, Wada J, Horiuchi S, Makino H (1999) Advanced glycation end products-cytokine-nitric oxide sequence pathway in the development of diabetic nephropathy: aminoguanidine ameliorates the overexpression of tumour necrosis factor-alpha and inducible nitric oxide synthase in diabetic rat glomeruli. Diabetologia 42:878–886
Wautier JL (2004) Protein glycation: a firm link to endothelial cell dysfunction. Circ Res 95(3):233–238. doi:10.1161/01.res.0000137876.28454.64
Candido R (2003) A breaker of advanced glycation end products attenuates diabetes-induced myocardial structural changes. Circ Res 92(7):785–792. doi:10.1161/01.res.0000065620.39919.20
Lee HB (2003) Reactive oxygen species-regulated signaling pathways in diabetic nephropathy. J Am Soc Nephrol 14(90003):S241–S245. doi:10.1097/01.asn.0000077410.66390.0f
Wu F, Tyml K, Wilson JX (2008) iNOS expression requires NADPH oxidase-dependent redox signaling in microvascular endothelial cells. J Cell Physiol 217(1):207–214. doi:10.1002/jcp.21495
Fan Q, Liao J, Kobayashi M, Yamashita M, Gu L, Gohda T, Suzuki Y, Wang LN, Horikoshi S, Tomino Y (2004) Candesartan reduced advanced glycation end-products accumulation and diminished nitro-oxidative stress in type 2 diabetic KK/Ta mice. Nephrol Dial Transplant 19(12):3012–3020. doi:10.1093/ndt/gfh499
Youn JY, Gao L, Cai H (2012) The p47phox- and NADPH oxidase organiser 1 (NOXO1)-dependent activation of NADPH oxidase 1 (NOX1) mediates endothelial nitric oxide synthase (eNOS) uncoupling and endothelial dysfunction in a streptozotocin-induced murine model of diabetes. Diabetologia 55(7):2069–2079. doi:10.1007/s00125-012-2557-6
Acknowledgments
This study is supported by the National Natural Science Foundation of China (Project # 81170767, Dr. Gao).
Author information
Authors and Affiliations
Corresponding author
Additional information
Gao and Huang contribute equally to the paper.
Rights and permissions
About this article
Cite this article
Gao, L., Huang, W. & Li, J. NOX1 abet mesangial fibrogenesis via iNOS induction in diabetes. Mol Cell Biochem 382, 185–191 (2013). https://doi.org/10.1007/s11010-013-1733-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-013-1733-4