Abstract
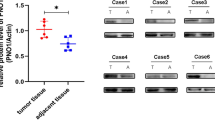
TRAF6, a unique tumor necrosis factor receptor-associated factor (TRAF) family member, possesses a unique receptor-binding specificity that results in its crucial role as the signaling mediator for TNF receptor superfamily and interleukin-1 receptor/Toll-like receptor superfamily. TRAF6 plays an important role in tumorigenesis, invasion and metastasis. This study aimed to explore the expression of TRAF6 in osteosarcoma tissues and its correlation to the clinical pathology of osteosarcoma and to discuss the relationship between TRAF6 expression and osteosarcoma invasion. These data will provide the experimental base for the biological treatment of osteosarcoma in the future. Using RT-PCR and Western blot, the results showed that the expression rate of TRAF6 mRNA in osteosarcoma tissues was significantly higher than that in normal bone tissue (p < 0.05), that the expression rate of TRAF6 mRNA in the carcinoma tissues from patients with lung metastasis was significantly higher than that from patients without lung metastasis (p < 0.05), and that the expression rate of TRAF6 mRNA also increased with increasing Enneking stage (p < 0.05). However, the mRNA expression of TRAF6 in osteosarcoma was independent of the patient’s gender, age, and tumor size (p > 0.05). The TRAF6 protein displayed an up-regulation in osteosarcoma tissues compared to normal bone tissue (p < 0.05), displayed an up-regulation in osteosarcoma tissues from patients with lung metastasis compared to from patients without lung metastasis (p < 0.05), and displayed a gradual increase with increasing Enneking stage (p < 0.05). By the technique of RNA interference, the expression of TRAF6 in the human osteosarcoma MG-63 cell line was down-regulated, and the invasive ability of MG-63 cells was examined. The results showed that TRAF6 protein expression was significantly decreased in the MG-63 cells from TRAF6 siRNA-transfected group (p < 0.05), and the proliferation ability of MG-63 cells and the number of MG-63 cells that passed through the Transwell chamber were significantly lower than that in the non-transfected control group as well as the transfected control group (p < 0.05). In addition, the percentage of MG-63 cells undergoing apoptosis was significantly higher in the TRAF6 siRNA-transfected group compared with the non-transfected control group as well as the transfected control group (p < 0.05). The expression of p-p65, cyclin D1, MMP-9 was down-regulated in the MG-63 cells from TRAF6 siRNA-transfected group. The expression of caspase 3 was up-regulated in the MG-63 cells from TRAF6 siRNA-transfected group compared to the non-transfected control group as well as the transfected control group (p < 0.05). To make a long story short, the overexpression of TRAF6 in osteosarcoma might be related to the tumorigenesis, invasion of osteosarcoma.






Similar content being viewed by others
References
PosthumaDeBoer J, Witlox MA, Kaspers GJ, van Royen BJ (2011) Molecular alterations as target for therapy in metastatic osteosarcoma: a review of literature. Clin Exp Metastasis 28:493–503
Bielack SS, Carrle D, Hardes J, Schuck A, Paulussen M (2008) Bone tumors in adolescents and young adults. Curr Treat Options Oncol 9:67–80
Harting MT, Blakely ML (2006) Management of osteosarcoma pulmonary metastases. Semin Pediatr Surg 15:25–29
Zwaga T, Bovee JV, Kroon HM (2008) Osteosarcoma of the femur with skip, lymph node, and lung metastases. Radiographics 28:277–283
Shen A, Zhang Y, Yang H, Xu R, Huang G (2012) Overexpression of ZEB1 relates to metastasis and invasion in osteosarcoma. J Surg Oncol 105:830–834
Tumbar T, Guasch G, Greco V, Blanpain C, Lowry WE, Rendl M, Fuchs E (2004) Defining the epithelial stem cell niche in skin. Science 303:359–363
Spradling A, Drummond-Barbosa D, Kai T (2001) Stem cells find their niche. Nature 414:98–104
Bradley JR, Pober JS (2001) Tumor necrosis factor receptor-associated factors (TRAFs). Oncogene 20:6482–6491
Wixted JH, Rothstein JL, Eisenlohr LC (2012) Identification of functionally distinct TRAF proinflammatory and phosphatidylinositol 3-kinase/mitogen-activated protein kinase/extracellular signal-regulated kinase kinase (PI3K/MEK) transforming activities emanating from RET/PTC fusion oncoprotein. J Biol Chem 287:3691–3703
Inoue J, Ishida T, Tsukamoto N, Kobayashi N, Naito A, Azuma S, Yamamoto T (2000) Tumor necrosis factor receptor-associated factor (TRAF) family: adapter proteins that mediate cytokine signaling. Exp Cell Res 254:14–24
Rothe M, Wong SC, Henzel WJ, Goeddel DV (1994) A novel family of putative signal transducers associated with the cytoplasmic domain of the 75 kDa tumor necrosis factor receptor. Cell 78:681–692
Avila M, Martinez-Juarez A, Ibarra-Sanchez A, Gonzalez-Espinosa C (2012) Lyn kinase controls TLR4-dependent IKK and MAPK activation modulating the activity of TRAF-6/TAK-1 protein complex in mast cells. Innate Immun 18:648–660
Ishida TK, Tojo T, Aoki T, Kobayashi N, Ohishi T, Watanabe T, Yamamoto T, Inoue J (1996) TRAF5, a novel tumor necrosis factor receptor-associated factor family protein, mediates CD40 signaling. Proc Natl Acad Sci USA 93:9437–9442
Ishida T, Mizushima S, Azuma S, Kobayashi N, Tojo T, Suzuki K, Aizawa S, Watanabe T, Mosialos G, Kieff E et al (1996) Identification of TRAF6, a novel tumor necrosis factor receptor-associated factor protein that mediates signaling from an amino-terminal domain of the CD40 cytoplasmic region. J Biol Chem 271:28745–28748
Xu LG, Li LY, Shu HB (2004) TRAF7 potentiates MEKK3-induced AP1 and CHOP activation and induces apoptosis. J Biol Chem 279:17278–17282
Xu Y, Cheng G, Baltimore D (1996) Targeted disruption of TRAF3 leads to postnatal lethality and defective T-dependent immune responses. Immunity 5:407–415
Regnier CH, Tomasetto C, Moog-Lutz C, Chenard MP, Wendling C, Basset P, Rio MC (1995) Presence of a new conserved domain in CART1, a novel member of the tumor necrosis factor receptor-associated protein family, which is expressed in breast carcinoma. J Biol Chem 270:25715–25721
Takeuchi M, Rothe M, Goeddel DV (1996) Anatomy of TRAF2. Distinct domains for nuclear factor-kappaB activation and association with tumor necrosis factor signaling proteins. J Biol Chem 271:19935–19942
Soni V, Cahir-McFarland E, Kieff E (2007) LMP1 TRAFficking activates growth and survival pathways. Adv Exp Med Biol 597:173–187
Rothe M, Sarma V, Dixit VM, Goeddel DV (1995) TRAF2-mediated activation of NF-kappa B by TNF receptor 2 and CD40. Science 269:1424–1427
Landstrom M (2010) The TAK1–TRAF6 signalling pathway. Int J Biochem Cell Biol 42:585–589
Lee SY, Choi Y (2007) TRAF1 and its biological functions. Adv Exp Med Biol 597:25–31
Naito A, Azuma S, Tanaka S, Miyazaki T, Takaki S, Takatsu K, Nakao K, Nakamura K, Katsuki M, Yamamoto T et al (1999) Severe osteopetrosis, defective interleukin-1 signalling and lymph node organogenesis in TRAF6-deficient mice. Genes Cells 4:353–362
Chung JY, Park YC, Ye H, Wu H (2002) All TRAFs are not created equal: common and distinct molecular mechanisms of TRAF-mediated signal transduction. J Cell Sci 115:679–688
Vaiopoulos AG, Papachroni KK, Papavassiliou AG (2010) Colon carcinogenesis: learning from NF-kappaB and AP-1. Int J Biochem Cell Biol 42:1061–1065
Cooper SJ, Bowden GT (2007) Ultraviolet B regulation of transcription factor families: roles of nuclear factor-kappa B (NF-kappaB) and activator protein-1 (AP-1) in UVB-induced skin carcinogenesis. Curr Cancer Drug Targets 7:325–334
King CG, Kobayashi T, Cejas PJ, Kim T, Yoon K, Kim GK, Chiffoleau E, Hickman SP, Walsh PT, Turka LA et al (2006) TRAF6 is a T cell-intrinsic negative regulator required for the maintenance of immune homeostasis. Nat Med 12:1088–1092
Lamothe B, Webster WK, Gopinathan A, Besse A, Campos AD, Darnay BG (2007) TRAF6 ubiquitin ligase is essential for RANKL signaling and osteoclast differentiation. Biochem Biophys Res Commun 359:1044–1049
Poblenz AT, Jacoby JJ, Singh S, Darnay BG (2007) Inhibition of RANKL-mediated osteoclast differentiation by selective TRAF6 decoy peptides. Biochem Biophys Res Commun 359:510–515
Ramachandran C, Rodriguez S, Ramachandran R, Raveendran Nair PK, Fonseca H, Khatib Z, Escalon E, Melnick SJ (2005) Expression profiles of apoptotic genes induced by curcumin in human breast cancer and mammary epithelial cell lines. Anticancer Res 25:3293–3302
Starczynowski DT, Lockwood WW, Delehouzee S, Chari R, Wegrzyn J, Fuller M, Tsao MS, Lam S, Gazdar AF, Lam WL et al (2011) TRAF6 is an amplified oncogene bridging the RAS and NF-kappaB pathways in human lung cancer. J Clin Invest 121:4095–4105
Ma T, Wang N, Su Z, Chen L, Zhu N, Ma C, Chen X, Chen H (2011) Characterization of apoptosis and proliferation in esophageal carcinoma EC109 cells following siRNA-induced down-regulation of TRAF6. Mol Cell Biochem 352:77–85
Liu H, Tamashiro S, Baritaki S, Penichet M, Yu Y, Chen H, Berenson J, Bonavida B (2012) TRAF6 activation in multiple myeloma: a potential therapeutic target. Clin Lymphoma Myeloma Leuk 12:155–163
Chaudhry SI, Hooper S, Nye E, Williamson P, Harrington K, Sahai E (2012) Autocrine IL-1beta-TRAF6 signalling promotes squamous cell carcinoma invasion through paracrine TNFalpha signalling to carcinoma-associated fibroblasts. Oncogene. doi:10.1038/onc.2012.1091onc201291
Enneking WF, Spanier SS, Goodman MA (1980) A system for the surgical staging of musculoskeletal sarcoma. Clin Orthop Relat Res 153:106–120
Ben-David D, Livne E, Reznick AZ (2012) The involvement of oxidants and NF-kappaB in cytokine-induced MMP-9 synthesis by bone marrow-derived osteoprogenitor cells. Inflamm Res 61:673–688
Wang SJ, Sun B, Pan SH, Chen H, Kong R, Li J, Xue DB, Bai XW, Jiang HC (2010) Experimental study of the function and mechanism combining dihydroartemisinin and gemcitabine in treating pancreatic cancer. Zhonghua Wai Ke Za Zhi 48:530–534
Wang YW, Wang SJ, Zhou YN, Pan SH, Sun B (2012) Escin augments the efficacy of gemcitabine through down-regulation of nuclear factor-kappaB and nuclear factor-kappaB-regulated gene products in pancreatic cancer both in vitro and in vivo. J Cancer Res Clin Oncol 138:785–797
Ilvesaro JM, Merrell MA, Li L, Wakchoure S, Graves D, Brooks S, Rahko E, Jukkola-Vuorinen A, Vuopala KS, Harris KW et al (2008) Toll-like receptor 9 mediates CpG oligonucleotide-induced cellular invasion. Mol Cancer Res 6:1534–1543
Starska K, Forma E, Brys M, Glowacka E, Lewy-Trenda I, Lukomski M, Krajewska WM (2012) The expression of TLR pathway molecules in peripheral blood mononuclear cells and their relationship with tumor invasion and cytokine secretion in laryngeal carcinoma. Adv Med Sci 57:124–135
Chen YY, Chiang SY, Lin JG, Ma YS, Liao CL, Weng SW, Lai TY, Chung JG (2010) Emodin, aloe-emodin and rhein inhibit migration and invasion in human tongue cancer SCC-4 cells through the inhibition of gene expression of matrix metalloproteinase-9. Int J Oncol 36:1113–1120
Kim A, Kim MJ, Yang Y, Kim JW, Yeom YI, Lim JS (2009) Suppression of NF-kappaB activity by NDRG2 expression attenuates the invasive potential of highly malignant tumor cells. Carcinogenesis 30:927–936
Argast GM, Krueger JS, Thomson S, Sujka-Kwok I, Carey K, Silva S, O’Connor M, Mercado P, Mulford IJ, Young GD et al (2011) Inducible expression of TGFbeta, snail and Zeb1 recapitulates EMT in vitro and in vivo in a NSCLC model. Clin Exp Metastasis 28:593–614
Author information
Authors and Affiliations
Corresponding author
Additional information
Qingbing Meng and Minqian Zheng contributed equally to this work.
Rights and permissions
About this article
Cite this article
Meng, Q., Zheng, M., Liu, H. et al. TRAF6 regulates proliferation, apoptosis, and invasion of osteosarcoma cell. Mol Cell Biochem 371, 177–186 (2012). https://doi.org/10.1007/s11010-012-1434-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-012-1434-4