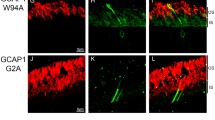
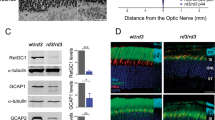
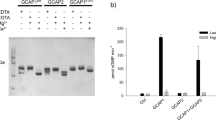
Abstract
Targeted deletion of membrane guanylate cyclases (GCs) has yielded new information concerning their function. Here, we summarize briefly recent results of laboratory generated non-photoreceptor GC knockouts characterized by complex phenotypes affecting the vasculature, heart, brain, kidney, and other tissues. The main emphasis of the review, however, addresses the two GCs expressed in retinal photoreceptors, termed GC-E and GC-F. Naturally occurring GC-E (GUCY2D) null alleles in human and chicken are associated with an early onset blinding disorder, termed “Leber congenital amaurosis type 1” (LCA-1), characterized by extinguished scotopic and photopic ERGs, and retina degeneration. In mouse, a GC-E null genotype produces a recessive cone dystrophy, while rods remain functional. Rod function is supported by the presence of GC-F (Gucy2f), a close relative of GC-E. Deletion of Gucy2f has very little effect on rod and cone physiology and survival. However, a GC-E/GC-F double knockout (GCdko) phenotypically resembles human LCA-1 with extinguished ERGs and rod/cone degeneration. In GCdko rods, PDE6 and GCAPs are absent in outer segments. In contrast, GC-E−/− cones lack proteins of the entire phototransduction cascade. These results suggest that GC-E may participate in transport of peripheral membrane proteins from the endoplasmic reticulum (ER) to the outer segments.








Similar content being viewed by others
Abbreviations
- ER:
-
Endoplasmic reticulum
- GC:
-
Guanylate cyclase
- GCdko:
-
GC-E/GC-F double knockout
- GCAP:
-
Guanylate cyclase-activating protein
- LCA:
-
Leber congenital amaurosis
- PDE6:
-
cGMP-specific photoreceptor phosphodiesterase
- TGN:
-
trans-Golgi network
- WT:
-
Wild-type
References
Kuhn M (2003) Structure, regulation, and function of mammalian membrane guanylyl cyclase receptors, with a focus on guanylyl cyclase-A. Circ Res 93:700–709
Lucas KA, Pitari GM, Kazerounian S, Ruiz-Stewart I, Park J, Schulz S, Chepenik KP, Waldman SA (2000) Guanylyl cyclases and signaling by cyclic GMP. Pharmacol Rev 52:375–414
Potter LR (2005) Domain analysis of human transmembrane guanylyl cyclase receptors: implications for regulation. Front Biosci 10:1205–1220
Sitaramayya A (2002) Soluble guanylate cyclases in the retina. Mol Cell Biochem 230:177–186
Noll GN, Billek M, Pietruck C, Schmidt KF (1994) Inhibition of nitric oxide synthase alters light responses and dark voltage of amphibian photoreceptors. Neuropharmacology 33:1407–1412
Cao L, Blute TA, Eldred WD (2000) Localization of heme oxygenase-2 and modulation of cGMP levels by carbon monoxide and/or nitric oxide in the retina. Vis Neurosci 17:319–329
Garbers DL, Chrisman TD, Wiegn P, Katafuchi T, Albanesi JP, Bielinski V, Barylko B, Redfield MM, Burnett JC Jr (2006) Membrane guanylyl cyclase receptors: an update. Trends Endocrinol Metab 17:251–258
Kuhn M (2005) Cardiac and intestinal natriuretic peptides: insights from genetically modified mice. Peptides 26:1078–1085
Lopez MJ, Wong SK, Kishimoto I, Dubois S, Mach V, Friesen J, Garbers DL, Beuve A (1995) Salt-resistant hypertension in mice lacking the guanylyl cyclase-A receptor for atrial natriuretic peptide. Nature 378:65–68
Oliver PM, Fox JE, Kim R, Rockman HA, Kim HS, Reddick RL, Pandey KN, Milgram SL, Smithies O, Maeda N (1997) Hypertension, cardiac hypertrophy, and sudden death in mice lacking natriuretic peptide receptor A. Proc Natl Acad Sci USA 94:14730–14735
Tamura N, Doolittle LK, Hammer RE, Shelton JM, Richardson JA, Garbers DL (2004) Critical roles of the guanylyl cyclase B receptor in endochondral ossification and development of female reproductive organs. Proc Natl Acad Sci USA 101:17300–17305
Olney RC, Bukulmez H, Bartels CF, Prickett TC, Espiner EA, Potter LR, Warman ML (2006) Heterozygous mutations in natriuretic peptide receptor-B (NPR2) are associated with short stature. J Clin Endocrinol Metab 91:1229–1232
Sogawa C, Tsuji T, Shinkai Y, Katayama K, Kunieda T (2007) Short-limbed dwarfism: slw is a new allele of Npr2 causing chondrodysplasia. J Hered 98:575–580
Tsuji T, Kunieda T (2005) A loss-of-function mutation in natriuretic peptide receptor 2 (Npr2) gene is responsible for disproportionate dwarfism in cn/cn mouse. J Biol Chem 280:14288–14292
Schulz S, Lopez MJ, Kuhn M, Garbers DL (1997) Disruption of the guanylyl cyclase-C gene leads to a paradoxical phenotype of viable but heat-stable enterotoxin-resistant mice. J Clin Invest 100:1590–1595
Mann EA, Steinbrecher KA, Stroup C, Witte DP, Cohen MB, Giannella RA (2005) Lack of guanylyl cyclase C, the receptor forEscherichia coli heat-stable enterotoxin, results in reduced polyp formation and increased apoptosis in the multiple intestinal neoplasia (Min) mouse model. Int J Cancer 116:500–505
Juilfs DM, Fulle HJ, Zhao AZ, Houslay MD, Garbers DL, Beavo JA (1997) A subset of olfactory neurons that selectively express cGMP-stimulated phosphodiesterase (PDE2) and guanylyl cyclase-D define a unique olfactory signal transduction pathway. Proc Natl Acad Sci USA 94:3388–3395
Leinders-Zufall T, Cockerham RE, Michalakis S, Biel M, Garbers DL, Reed RR, Zufall F, Munger SD (2007) Contribution of the receptor guanylyl cyclase GC-D to chemosensory function in the olfactory epithelium. Proc Natl Acad Sci USA 104:14507–14512
Duda T, Sharma RK (2008) ONE-GC membrane guanylate cyclase, a trimodal odorant signal transducer. Biochem Biophys Res Commun 367:440–445
Kuhn M, Ng CK, Su YH, Kilic A, Mitko D, Bien-Ly N, Komuves LG, Yang RB (2004) Identification of an orphan guanylate cyclase receptor selectively expressed in mouse testis. Biochem J 379:385–393
Lin H, Cheng CF, Hou HH, Lian WS, Chao YC, Ciou YY, Djoko B, Tsai MT, Cheng CJ, Yang RB (2008) Disruption of guanylyl cyclase-G protects against acute renal injury. J Am Soc Nephrol 19:339–348
Young JM, Waters H, Dong C, Fulle HJ, Liman ER (2007) Degeneration of the olfactory guanylyl cyclase D gene during primate evolution. PLoS ONE 2:e884
Polans A, Baehr W, Palczewski K (1996) Turned on by Ca2+! The physiology and pathology of Ca2+ binding proteins in the retina. Trends Neurosci 19:547–554
Wensel TG (2008) Signal transducing membrane complexes of photoreceptor outer segments. Vis Res 48:2052–2061
Lamb TD, Pugh EN Jr (2006) Phototransduction, dark adaptation, and rhodopsin regeneration the proctor lecture. Invest Ophthalmol Vis Sci 47:5138–5152
Arshavsky VY, Lamb TD, Pugh EN Jr (2002) G proteins and phototransduction. Annu Rev Physiol 64:153–187
Shyjan AW, de Sauvage FJ, Gillett NA, Goeddel DV, Lowe DG (1992) Molecular cloning of a retina-specific membrane guanylyl cyclase. Neuron 9:727–737
Garbers DL, Lowe DG (1994) Guanylyl cyclase receptors. J Biol Chem 269:30741–30744
Pugh EN Jr, Duda T, Sitaramayya A, Sharma RK (1997) Photoreceptor guanylate cyclases: a review. Biosci Rep 17:429–473
Koch KW, Duda T, Sharma RK (2002) Photoreceptor specific guanylate cyclases in vertebrate phototransduction. Mol Cell Biochem 230:97–106
Baehr W, Karan S, Maeda T, Luo DG, Li S, Bronson JD, Watt CB, Yau KW, Frederick JM, Palczewski K (2007) The function of guanylate cyclase 1 and guanylate cyclase 2 in rod and cone photoreceptors. J Biol Chem 282:8837–8847
Liu Q, Tan G, Levenkova N, Li T, Pugh EN Jr, Rux JJ, Speicher DW, Pierce EA (2007) The proteome of the mouse photoreceptor sensory cilium complex. Mol Cell Proteomics 6:1299–1317
Helten A, Saftel W, Koch KW (2007) Expression level and activity profile of membrane bound guanylate cyclase type 2 in rod outer segments. J Neurochem 103:1439–1446
Dizhoor AM, Lowe DG, Olshevskaya EV, Laura RP, Hurley JB (1994) The human photoreceptor membrane guanylyl cyclase, RetGC, is present in outer segments and is regulated by Calcium and a soluble activator. Neuron 12:1345–1352
Lowe DG, Dizhoor AM, Liu K, Gu Q, Spencer M, Laura R, Lu L, Hurley JB (1995) Cloning and expression of a second photoreceptor-specific membrane retina guanylyl cyclase (RetGC), RetGC-2. Proc Natl Acad Sci USA 92:5535–5539
Imanishi Y, Li N, Sowa ME, Lichtarge O, Wensel TG, Saperstein DA, Baehr W, Palczewski K (2002) Characterization of retinal guanylate cyclase-activating protein 3 (GCAP3) from zebrafish to man. Eur J Neurosci 15:63–78
Liu X, Seno K, Nishizawa Y, Hayashi F, Yamazaki A, Matsumoto H, Wakabayashi T, Usukura J (1994) Ultrastructural localization of retinal guanylate cyclase in human and monkey retinas. Exp Eye Res 59:761–768
Yang RB, Garbers DL (1997) Two eye guanylyl cyclases are expressed in the same photoreceptor cells and form homomers in preference to heteromers. J Biol Chem 272:13738–13742
Haire SE, Pang J, Boye SL, Sokal I, Craft CM, Palczewski K, Hauswirth WW, Semple-Rowland SL (2006) Light-driven cone arrestin translocation in cones of postnatal guanylate cyclase-1 knockout mouse retina treated with AAV-GC1. Invest Ophthalmol Vis Sci 47:3745–3753
Duda T, Koch KW, Venkataraman V, Lange C, Beyermann M, Sharma RK (2002) Ca(2+) sensor S100beta-modulated sites of membrane guanylate cyclase in the photoreceptor-bipolar synapse. EMBO J 21:2547–2556
Venkataraman V, Duda T, Vardi N, Koch KW, Sharma RK (2003) Calcium-modulated guanylate cyclase transduction machinery in the photoreceptor–bipolar synaptic region. Biochemistry 42:5640–5648
Venkataraman V, Nagele R, Duda T, Sharma RK (2000) Rod outer segment membrane guanylate cyclase type 1-linked stimulatory and inhibitory calcium signaling systems in the pineal gland: biochemical, molecular, and immunohistochemical evidence. Biochemistry 39:6042–6052
Duda T, Venkataraman V, Krishnan A, Nagele RG, Sharma RK (2001) Negatively calcium-modulated membrane guanylate cyclase signaling system in the rat olfactory bulb. Biochemistry 40:4654–4662
Jankowska A, Burczynska B, Duda T, Warchol JB, Sharma RK (2007) Calcium-modulated rod outer segment membrane guanylate cyclase type 1 transduction machinery in the testes. J Androl 28:50–58
Seebacher T, Beitz E, Kumagami H, Wild K, Ruppersberg JP, Schultz JE (1999) Expression of membrane-bound and cytosolic guanylyl cyclases in the rat inner ear. Hear Res 127:95–102
Shao RX, Otsuka M, Kato N, Chang JH, Muroyama R, Taniguchi H, Moriyama M, Wang Y, Kawabe T, Omata M (2005) No mutations in the tyrosine kinases of human hepatic, pancreatic, and gastric cancer cell lines. J Gastroenterol 40:918
Palczewski K, Polans AS, Baehr W, Ames JB (2000) Calcium binding proteins in the retina: structure, function, and the etiology of human visual diseases. Bioessays 22:337–350
Sokal I, Li N, Verlinde CL, Haeseleer F, Baehr W, Palczewski K (2000) Ca2+-binding proteins in the retina: from discovery to etiology of human disease. Biochim Biophys Acta 1498:233–251
Dizhoor AM, Hurley JB (1999) Regulation of photoreceptor membrane guanylyl cyclases by guanylyl cyclase activator proteins. Methods 19:521–531
Koch KW (2002) Target recognition of guanylate cyclase by guanylate cyclase-activating proteins. Adv Exp Med Biol 514:349–360
Duda T, Goraczniak RM, Sharma RK (1996) Molecular characterization of S100A1–S100B protein in retina and its activation mechanism of bovine photoreceptor guanylate cyclase. Biochemistry 35:6263–6266
Kumar VD, Vijay-Kumar S, Krishnan A, Duda T, Sharma RK (1999) A second calcium regulator of rod outer segment membrane guanylate cyclase, ROS-GC1: neurocalcin. Biochemistry 38:12614–12620
Goraczniak RM, Duda T, Sitaramayya A, Sharma RK (1994) Structural and functional characterization of the rod outer segment membrane guanylate cyclase. Biochem J 302:455–461
Perrault I, Hanein S, Gerber S, Lebail B, Vlajnik P, Barbet F, Ducroq D, Dufier JL, Munnich A, Kaplan J, Rozet JM (2005) A novel mutation in the GUCY2D gene responsible for an early onset severe RP different from the usual GUCY2D-LCA phenotype. Hum Mutat 25:222
Yang RB, Fulle HJ, Garbers DL (1996) Chromosomal localization and genomic organization of genes encoding guanylyl cyclase receptors expressed in olfactory sensory neurons and retina. Genomics 31:367–372
Wood LD, Calhoun ES, Silliman N, Ptak J, Szabo S, Powell SM, Riggins GJ, Wang TL, Yan H, Gazdar A, Kern SE, Pennacchio L, Kinzler KW, Vogelstein B, Velculescu VE (2006) Somatic mutations of GUCY2F, EPHA3, and NTRK3 in human cancers. Hum Mutat 27:1060–1061
Perrault I, Rozet JM, Gerber S, Ghazi I, Ducroq D, Souied E, Leowski C, Bonnemaison M, Dufier JL, Munnich A, Kaplan J (2000) Spectrum of retGC1 mutations in Leber’s congenital amaurosis. Eur J Hum Genet 8:578–582
Perrault I, Rozet J-M, Calvas P, Gerber S, Camuzat A, Dollfus H, Chatelin S, Souied E, Ghazi I, Leowski C, Bonnermaison M, Le Paslier D, Frezal J, Dufier J-L, Pittler SJ, Munnich A, Kaplan J (1996) Retinal-specific guanylate cyclase gene mutations in Leber’s congenital amaurosis. Nat Genet 14:461–464
Yzer S, Leroy BP, De BE, de Ravel TJ, Zonneveld MN, Voesenek K, Kellner U, Ciriano JP, de Faber JT, Rohrschneider K, Roepman R, den Hollander AI, Cruysberg JR, Meire F, Casteels I, van Moll-Ramirez NG, Allikmets R, Van den Born LI, Cremers FP (2006) Microarray-based mutation detection and phenotypic characterization of patients with Leber congenital amaurosis. Invest Ophthalmol Vis Sci 47:1167–1176
Milam AH, Barakat MR, Gupta N, Rose L, Aleman TS, Pianta MJ, Cideciyan AV, Sheffield VC, Stone EM, Jacobson SG (2003) Clinicopathologic effects of mutant GUCY2D in Leber congenital amaurosis. Ophthalmology 110:549–558
Tucker CL, Ramamurthy V, Pina AL, Loyer M, Dharmaraj S, Li Y, Maumenee IH, Hurley JB, Koenekoop RK (2004) Functional analyses of mutant recessive GUCY2D alleles identified in Leber congenital amaurosis patients: protein domain comparisons and dominant negative effects. Mol Vis 10:297–303
Kelsell RE, Gregory-Evans K, Payne AM, Perrault I, Kaplan J, Yang R-B, Garbers DL, Bird AC, Moore AT, Hunt DF (1998) Mutations in the retinal guanylate cyclase (RETGC-1) gene in dominant con-rod dystrophy. Hum Mol Genet 7:1179–1184
Perrault I, Rozet JM, Gerber S, Kelsell RE, Souied E, Cabot A, Hunt DM, Munnich A, Kaplan J (1998) A retGC-1 mutation in autosomal dominant cone-rod dystrophy. Am J Hum Genet 63:651–654
Payne AM, Morris AG, Downes SM, Johnson S, Bird AC, Moore AT, Bhattacharya SS, Hunt DM (2001) Clustering and frequency of mutations in the retinal guanylate cyclase (GUCY2D) gene in patients with dominant cone-rod dystrophies. J Med Genet 38:611–614
Downes SM, Payne AM, Kelsell RE, Fitzke FW, Holder GE, Hunt DM, Moore AT, Bird AC (2001) Autosomal dominant cone-rod dystrophy with mutations in the guanylate cyclase 2D gene encoding retinal guanylate cyclase-1. Arch Ophthalmol 119:1667–1673
Wilkie SE, Newbold RJ, Deery E, Walker CE, Stinton I, Ramamurthy V, Hurley JB, Bhattacharya SS, Warren MJ, Hunt DM (2000) Functional characterization of missense mutations at codon 838 in retinal guanylate cyclase correlates with disease severity in patients with autosomal dominant cone-rod dystrophy. Hum Mol Genet 9:3065–3073
Tucker CL, Woodcock SC, Kelsell RE, Ramamurthy V, Hunt DM, Hurley JB (1999) Biochemical analysis of a dimerization domain mutation in RetGC-1 associated with dominant cone-rod dystrophy. Proc Natl Acad Sci USA 96:9039–9044
Ramamurthy V, Tucker C, Wilkie SE, Daggett V, Hunt DM, Hurley JB (2001) Interactions within the coiled-coil domain of RetGC-1 guanylyl cyclase are optimized for regulation rather than for high affinity. J Biol Chem 276:26218–26229
Semple-Rowland SL, Cheng KM (1999) rd and rc chickens carry the same GC1 null allele (GUCY1*). Exp Eye Res 69:579–581
Semple-Rowland SL, Lee NR, Van-Hooser JP, Palczewski K, Baehr W (1998) A null mutation in the photoreceptor guanylate cyclase gene causes the retinal degeneration chicken phenotype. Proc Natl Acad Sci USA 95:1271–1276
Semple-Rowland SL, Lee NR (2000) Avian models of inherited retinal disease. Methods Enzymol 316:526–536
Hanein S, Perrault I, Gerber S, Tanguy G, Barbet F, Ducroq D, Calvas P, Dollfus H, Hamel C, Lopponen T, Munier F, Santos L, Shalev S, Zafeiriou D, Dufier JL, Munnich A, Rozet JM, Kaplan J (2004) Leber congenital amaurosis: comprehensive survey of the genetic heterogeneity, refinement of the clinical definition, and genotype-phenotype correlations as a strategy for molecular diagnosis. Hum Mutat 23:306–317
Williams ML, Coleman JE, Haire SE, Aleman TS, Cideciyan AV, Sokal I, Palczewski K, Jacobson SG, Semple-Rowland SL (2006) Lentiviral expression of retinal guanylate cyclase-1 (RetGC1) restores vision in an avian model of childhood blindness. PLoS Med 3:e201
Semple-Rowland SL, Green DA (1994) Molecular characterization of the α’-subunit of cone photoreceptor cGMP phosphodiesterase in normal and rd chicken. Exp Eye Res 59:365–372
Semple-Rowland SL, Green DA (1994) Molecular and biochemical analyses of Iodopsin in rd chick retina. Invest Ophthalmol Vis Sci 35:2550–2557
Semple-Rowland S, Gorczyca WA, Buczylko J, Ruiz CC, Subbaraya I, Helekar BS, Palczewski K, Baehr W (1996) Expression of GCAP1 and GCAP2 in the retinal degeneration (rd) chicken retina. FEBS Lett 385:47–52
Semple-Rowland SL, Larkin P, Bronson JD, Nykamp K, Streit WJ, Baehr W (1999) Characterization of the chicken GCAP gene array and analyses of GCAP1, GCAP2, and GC1 gene expression in normal and rd chicken pineal. Mol Vis 5:14
Yang RB, Robinson SW, Xiong WH, Yau KW, Birch DG, Garbers DL (1999) Disruption of a retinal guanylyl cyclase gene leads to cone-specific dystrophy and paradoxical rod behavior. J Neurosci 19:5889–5897
Coleman JE, Zhang Y, Brown GA, Semple-Rowland SL (2004) Cone cell survival and downregulation of GCAP1 protein in the retinas of GC1 knockout mice. Invest Ophthalmol Vis Sci 45:3397–3403
Anant JS, Ong OC, Xie H, Clarke S, O’Brien PJ, Fung BKK (1992) In vivo differential prenylation of retinal cyclic GMP phosphodiesterase catalytic subunits. J Biol Chem 267:687–690
Qin N, Pittler SJ, Baehr W (1992) In vitro isoprenylation and membrane association of mouse rod photoreceptor cGMP phosphodiesterase α and β subunits expressed in bacteria. J Biol Chem 267:8458–8463
Tai AW, Chuang JZ, Bode C, Wolfrum U, Sung CH (1999) Rhodopsin’s carboxy-terminal cytoplasmic tail acts as a membrane receptor for cytoplasmic dynein by binding to the dynein light chain Tctex-1. Cell 97:877–887
Deretic D (2006) A role for rhodopsin in a signal transduction cascade that regulates membrane trafficking and photoreceptor polarity. Vis Res 46:4427–4433
Zhang H, Li S, Doan T, Rieke F, Detwiler PB, Frederick JM, Baehr W (2007) Deletion of PrBP/{delta} impedes transport of GRK1 and PDE6 catalytic subunits to photoreceptor outer segments. Proc Natl Acad Sci USA 104:8857–8862
Karan S, Zhang H, Li S, Frederick JM, Baehr W (2008) A model for transport of membrane-associated phototransduction polypeptides in rod and cone photoreceptor inner segments. Vis Res 48:442–452
Insinna C, Besharse JC (2008) Intraflagellar transport and the sensory outer segment of vertebrate photoreceptors. Dev Dyn 237:1982–1992
Pazour GJ, Baker SA, Deane JA, Cole DG, Dickert BL, Rosenbaum JL, Witman GB, Besharse JC (2002) The intraflagellar transport protein, IFT88, is essential for vertebrate photoreceptor assembly and maintenance. J Cell Biol 157:103–113
Coleman JE, Semple-Rowland SL (2005) GC1 deletion prevents light-dependent arrestin translocation in mouse cone photoreceptor cells. Invest Ophthalmol Vis Sci 46:12–16
Fan J, Rohrer B, Frederick JM, Baehr W, Crouch RK (2008) Rpe65−/− and Lrat−/− mice: comparable models of Leber congenital amaurosis. Invest Ophthalmol Vis Sci 49:2384–2389
Zhang H, Fan J, Li S, Karan S, Rohrer B, Palczewski K, Frederick JM, Crouch RK, Baehr W (2008) Trafficking of membrane-associated proteins to cone photoreceptor outer segments requires the chromophore 11-cis-retinal. J Neurosci 28:4008–4014
Avasthi P, Watt CB, Williams DS, Le YZ, Li S, Chen C-K, Marc RE, Frederick JM, Baehr W (2009) Trafficking of membrane proteins to cone but not rod outer segments is dependent on heterotrimeric kinesin-II. J Neurosci 29:14287–14298
Lin JH, Li H, Yasumura D, Cohen HR, Zhang C, Panning B, Shokat KM, LaVail MM, Walter P (2007) IRE1 signaling affects cell fate during the unfolded protein response. Science 318:944–949
Rozet JM, Perrault I, Gerber S, Hanein S, Barbet F, Ducroq D, Souied E, Munnich A, Kaplan J (2001) Complete abolition of the retinal-specific guanylyl cyclase (retGC-1) catalytic ability consistently leads to Leber congenital amaurosis (LCA). Invest Ophthalmol Vis Sci 42:1190–1192
Lotery AJ, Namperumalsamy P, Jacobson SG, Weleber RG, Fishman GA, Musarella MA, Hoyt CS, Heon E, Levin A, Jan J, Lam B, Carr RE, Franklin A, Radha S, Andorf JL, Sheffield VC, Stone EM (2000) Mutation analysis of 3 genes in patients with Leber congenital amaurosis. Arch Ophthalmol 118:538–543
Duda T, Venkataraman V, Goraczniak R, Lange C, Koch KW, Sharma RK (1999) Functional consequences of a rod outer segment membrane guanylate cyclase (ROS-GC1) gene mutation linked with Leber’s congenital amaurosis. Biochemistry 38:509–515
El-Shanti H, Al-Salem M, El-Najjar M, Ajlouni K, Beck J, Sheffiled VC, Stone EM (1999) A nonsense mutation in the retinal specific guanylate cyclase gene is the cause of Leber congenital amaurosis in a large inbred kindred from Jordan. J Med Genet 36:862–865
Acknowledgments
This study was supported by RO1 EY08123, RO1 EY019298, P30 EY014800 and a Center Grant from the Foundation Fighting Blindness.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Karan, S., Frederick, J.M. & Baehr, W. Novel functions of photoreceptor guanylate cyclases revealed by targeted deletion. Mol Cell Biochem 334, 141–155 (2010). https://doi.org/10.1007/s11010-009-0322-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-009-0322-z