Abstract
Objectives
This study aimed to evaluate health related quality of life (HRQOL) in Egyptian children with systemic lupus erythematosus (SLE) using 3 different tools.
Methods
In this questionnaire-based study, 100 children with SLE were included. HRQOL was assessed using the Pediatric Quality of Life Inventory Generic Core Scales (PedsQL™ 4.0 GCS), PedsQL™ 3.0 Rheumatology Module (PedsQL3-RM) and the Simple Measure of the Impact of Lupus Erythematosus in Youngsters (SMILEY). SLE disease activity index (SLEDAI) was used to evaluate activity and SLE International Collaborating Clinics/ American College of Rheumatology Damage Index (SDI) was used to evaluate chronic damage.
Results
All mean scores of PedsQLTM4.0 GCS domains in SLE patients were lower than published normative data and previously published results of Egyptian healthy controls (p < 0.001). All mean scores of PedsQL-3RM domains were significantly lower than published normative data except for the treatment and pain and hurt domains (p = 0.1, 0.2 respectively). SMILEY scores were low and the lowest domain scores was “Burden of SLE”. Longer duration of illness, higher cumulative steroid doses, higher SLEDAI and SDI scores and presence of obesity were associated with lower scores for all 3 tools (p < 0.001).
Conclusion for Practice
The Arabic copies of PedsQL™ 4.0 GCS, PedsQL3-RM and SMILEY are easily used for Arabic speaking subjects and easily interpreted by physician and can be implemented for frequent monitoring of SLE HRQOL. Controlling the disease activity and using lowest doses of steroids and other immunosuppressive drugs are the corner stone strategies for improving HRQOL in SLE children.
Significance
This is the first study to evaluate HRQOL scores in Egyptian children with SLE which were found to be lower than published data with long disease duration, high cumulative disease activity, use of steroids and presence of obesity are the main influential factors related to low QoL scores. Generic and disease specific questionnaires are easily used for Arabic speaking subjects and easily interpreted by physician and can be implemented for frequent monitoring of SLE HRQOL. Controlling the disease activity and using lowest doses of steroids and other immunosuppressive drugs are the corner stone strategies for improving HRQOL in SLE children.
Similar content being viewed by others
Introduction
Childhood Systemic lupus erythematosus (cSLE) is characterized by severe course, widespread organ involvement especially renal and central nervous systems (CNS) and high mortality compared to adult-onset SLE (Ravelli et al., 2005). Young adults with SLE reported to have mortality rates 20- times higher than general population (Brunner et al., 2008). With the advances in treatment of cSLE, mortality rates decreased and life expectancy of the patients increased associated with a wide-ranging spectrum of long-term morbidities which accumulates more quickly in cSLE compared to adult-onset caused by higher rates of nephritis (Levy et al., 2014), more aggressive course which increases the exposure to immunosuppressive medications over a longer disease duration and the impact of disease and medications side effects on school performance and later on sexual and reproductive lives of affected adolescents (Hersh et al., 2009).
Health- related quality of life (HRQOL) has been defined as a “multi-dimensional concept including physical, social and psychological functioning related to a certain illness or its treatment” (Miller et al., 2002). The physical, emotional, and social effects of SLE drastically influence the QoL of patients. This can be related to repeated hospitalizations, follow up visits, frequent laboratory monitoring, health-care costs, and interference with daily activities (Hersh et al., 2009; Mina & Brunner, 2010). Research in adults with SLE suggests that the disease has a significant negative impact on the patients’ HRQOL (Thumboo & Strand, 2007). Similar research in children is lacking as there are few studies examining the HRQOL in small cSLE cohorts using standardized instruments (Brunner et al., 2009; Moorthy et al., 2017).
So, evaluating the different domains of QoL of children with SLE using pediatric specific tools have become critical determinants of the disease impact (Brunner & Giannini, 2003; Moorthy et al., 2005; Ravelli et al., 2005). Considering the paucity of information of QoL in cSLE in general, and Egyptian children particularly, we conducted the present study. The objectives of our study were to assess the QoL in children aged 8–18 years with SLE using the Pediatric Quality of Life Inventory Generic Core Scales (PedsQL™ 4.0 GCS), the PedsQL™ 3.0 Rheumatology Module (PedsQL™ 3.0 RM) and the Simple Measure of the Impact of Lupus Erythematosus in Youngsters (SMILEY), compare it to that of available cohorts; and assess the relationship of QoL with SLE disease features, activity, and damage scores.
Subjects and Methods
Participants
This questionnaire-based study was conducted in Mansoura University Children’s Hospital in Egypt over the period of 12 months. The study was approved by the local ethical committee and all participants/ guardians gave their informed consent prior to their inclusion in the study. A standardized introduction on HRQOL was given to both patients and parents prior to fulfilment of the questionnaires.
Inclusion Criteria
All included children were diagnosed to have SLE after fulfilling the American College of Rheumatology (ACR) classification criteria (Hochberg, 1997) and with a duration of illness of more than 1-year, normal kidney function, with no active infection and not admitted to the hospital at time of enrolment in the study.
Exclusion Criteria
Children with disabilities, psychological problems or obesity that have been diagnosed before the onset of SLE and those with mixed connective tissue diseases were excluded.
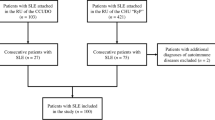
During the period of the study and after applying inclusion and exclusion criteria, 123 cSLE patients were approached to participate of which 100 were enrolled (23 patients not included as either refused to participate or agreed initially then did not return completed questionnaire form).
All patients with clinically evident lupus nephritis (LN) had ultrasound guided renal biopsy before starting treatment. Total cumulative steroid dose was calculated for patients’ weights and presented as g/kg according to which patients were divided into 2 groups: high cumulative glucocorticoids (CGCS) dose (> 1 gm/kg) and low CGCS (< 1 gm /kg). Obesity was defined as body mass index (BMI) above 95th percentile for age. Bone complications included: pathological fracture, avascular necrosis of femoral head and low bone mineral density (BMD) diagnosed as BMD z-score < -2 (El-Ziny et al., 2007).
Current and previously reported disease activity [evaluated by SLE Disease Activity Index 2000 (SLEDAI-2 K)] (Gladman et al., 2002) and disease related damage evaluated by SLE International Collaborating Clinics/ American College of Rheumatology Damage Index (SDI) were also obtained. SDI is an instrument that allows evaluation of accumulated damage in SLE patients including evaluation of 12 organ systems. Damage may be caused by previous disease activity or by medications and should be ascertained by clinical assessment and present for at least 6 months. A higher SDI score indicates worsening disease damage (Gladman et al., 1996). For comparative purposes, we used cutoff values like published articles to group the patients with greater disease activity and damage (cutoffs for SDI ≥ 2, SLEDAI ≥ 12) (Moorthy et al., 2007, 2009, 2017).
Procedures
All questionnaires were completed in paper form only. Questionnaire forms were completed during clinic visits. Questionnaires were completed by the children, with only few patients required some help from parents to understand some question (without guiding their answers).
Data were collected by a structured questionnaire using PedsQL™ 4.0 GCS, the PedsQL™ 3.0 RM, and the SMILEY module. An “Arabic for Egypt” version (Language of all participants) of the PedsQL™ 4.0 GCS is available for free after registration at (https://eprovide.mapi trust.org/instruments/pediatric-quality-of-life-inventory# need _ this _ questionnaire) provided by MAPI research institute, Lyon, France]. This version of the questionnaire has been previously used in the assessment of QoL in Egyptian children with nephrotic syndrome (NS) (Eid et al., 2020). The PedsQL™ 3.0 RM was translated into Arabic by the authors, and a professional English language expert. The Arabic copy of the questionnaire was approved by MAPI research institute, France and by professor James W. Varni. A free Arabic “for Egypt” copy of the SMILEY module was provided by Professor Moorthy with the permission to use it.
Measures
PedsQL™4.0 Generic Core Scale
We used the children (8–12 years) and adolescents (13–18 years) copies of the questionnaire according to patients’ age. A 5-point response scale was used from 0 to 4. Items are reverse-scored and converted to a 0–100 scale so higher scores indicate better QoL. Scale scores are computed as the sum of the items divided by the number of items answered (accounting for missing data). If more than 50% of the items in the scale are missing, the scale score is not computed (Varni et al., 2001). This accounts for the differences in sample sizes for scales reported in the results’ tables. The obtained results in all domains of PedsQL™ 4.0 were then compared to its previously published normative data (Varni et al., 2003) and results of 100 healthy control Egyptian children published in 2020 (Eid et al., 2020).
The PedsQL™ 3.0 RM
We used the children (8–12 years) and adolescents (13–18 years) copies of the questionnaire. The tool evaluates the QoL in 5 domains (pain and hurt, daily activities, treatment, worry, and communication). The format, instructions, Likert scale, and scoring scheme are the same as the PedsQL™ 4.0, with higher scores indicating better QoL (Varni et al., 2001). For our study, we used PedsQL™ 3.0 RM ratings of a cohort of 56 children with juvenile idiopathic arthritis (JIA) as normative values for comparison which was the first study to validate this rheumatology specific questionnaire in children with JIA being the most common pediatric rheumatology disorder (Brunner et al., 2004).
SMILEY
The SMILEY is the only disease-specific measure of HRQOL in cSLE. We used child report of the SMILEY measure. The SMILEY is a 26-item survey. The first 2 survey items are summary questions that are not included in the final score. Item 1 relates to current HRQOL status and item 2 relates to current SLE status. Responses are in the form of an illustrative 5-step scale with different facial expressions. If more than 12 questions are not answered, SMILEY cannot be scored. All items, including the first 2 summary questions, score from 1 to 5. The total score is transformed to a 1–100 scale (according to the scoring manual provided by Professor Moorthy). Higher scores reflect better QoL. Being a disease specific tool, there are no normative values for the SMILEY questionnaire (Moorthy et al., 2006).
Statistical Analysis
A sample size of 100 cSLE patients was chosen based on the duration of the study, availability of cases that fulfil inclusion criteria and collaboration of patients. Continuous variables with normal distribution were expressed as means and standard deviation and compared using Student’s t-test, whereas those not normally distributed were using Mann–Whitney U-test. Categorical variables were compared using the Chi-square test or Fisher’s exact test. Subsequently, the association of demographic parameters, clinical features, and disease complications to QoL scores was investigated for SLE patients (Table 6) using Pearson correlation or t-test (compare means) as relevant to the included variable. Correlation between SMILEY scores and each of PedsQL™ 4.0 GCS and PedsQL™ 3.0 RM scores was conducted. Statistical analysis was done using Statistical Package for the Social Science software version 25.0 (SPSS Inc., Chicago, IL, USA).
Results
One hundred children with SLE participated in the study, 79 females, and aged 14.04 ± 2.5 years at time of the study. Characteristics of patients are summarized in Table 1. All SLE children had normal kidney function at enrollment with eGFR 140.7 ± 25.8 ml/min/1.732. The number of medications taken by each patient at enrolment ranged between 2 and 5 medications including steroids, hydroxychloroquine, mycophenolate mofetil (MMF), cyclosporine, cyclophosphamide, antihypertensives, calcium and vitamin supplementation. Due to presence of missing data and as described in methodology section, total scores of the 3 used measures were calculated for 98 patients each. The mean scores for each domain and summary score of the 3 scales are presented in Tables 2 and 3. The mean scores for PedsQL™ 4.0 are compared to published normative data (Varni et al., 2003) and to the scores of 100 healthy Egyptian children data published in 2020 (Eid et al., 2020) showing significantly lower scores for SLE patients in all domains. The mean scores for PedsQL3-RM are compared to children diagnosed with JIA as a comparative population.
(Brunner et al., 2004) (Table 2) and showed significantly lower scores for in SLE patients compared to controls in all domains except for treatment and pain and hurt domains. Being a disease specific scale, SMILEY has no normative data, so we compared our results to published studies from different countries (Table 3).
As skin manifestations and LN are the most prevalent manifestations and LN being a leading cause of morbidities and mortalities and a unique feature of cSLE compared to other pediatric rheumatologic disorders and adult onset SLE, the total and domains’ scores for the used measures were compared between patients with and without LN and showed that SMILEY total and domain scores were significantly lower in patients with LN compared to those without LN, while no significant differences in PedsQL™ 4.0 scores and only worry domain of PedsQL3-RM was significantly lower in LN patients. Regarding skin manifestations, no significant differences reported in total and all domain scores of the 3 scales in patients with and without lupus skin manifestations. Disease activity was evaluated by SLEDAI score according to which patients were classified as having no/mild (SLEDAI < 12) (N = 77) or severe disease activity (SLEDAI ≥ 12((N = 23). The mean scores for all domains of the 3 scales for the 2 groups (mild and severe active diseases) were calculated and compared in Table 4. Disease related damage was evaluated by SDI according to which patients were classified as having no or minimal damage (SDI < 2) (N = 92) or severe damage (SDI ≥ 2) (N = 8). The mean scores for all domains of the 3 used scales for the 2 groups (no/mild and severe severe) were calculated and compared in Table 5. Table 6 shows correlation of QoL scores of the 3 measures used and different clinical manifestations and revealed that longer duration of illness, high cumulative steroid doses, higher SLEDAI and SDI scores, presence of obesity and low BMD were associated with low QoL scores for all 3 tools (p < 0.001). No significant differences were reported between male and female patients scores of all domains of 3 scales, disease activity or damage (p > 0.05 for all). Spearman correlation showed that SMILEY total scores corelated more strongly with PedsQL3-RM (r = 0.917, p < 0.001) than PedsQL™ 4.0 (r = 0.753, p < 0.001).
Discussion
The differences in cSLE regarding disease onset, phenotype, and outcomes between different ethnic groups is not fully elucidated (Massias et al., 2021). Additionally, differences in health care levels and attention to Qol also differ between countries. In Egypt the overall estimated prevalence of cSLE is 1/100,000 population (0.24/100000 males and 1.8/100000 females). The age of onset and female predominance are like other countries however, LN is more common in Egypt compared to cSLE from other countries (Eesa et al., 2021) which is associated with use of more immunosuppressives, frequent hospitalization and risk of deterioration of kidney functions. Youth with SLE in Egypt face many challenges including late diagnosis, cost of diagnostic work up, and treatment. Moreover, pediatric rheumatology/nephrology service is available only in few centers in Egypt. Some medications including recent biologics are not available in Egypt which limit choices available for refractory cases. Cyclophosphamide is used in up to 50% of cSLE in Egyptian patients due to its availability and low cost despite being not preferred by the patients and families due to cosmetic and gonadal side effects (Eesa et al., 2021 and Elmougy et al., 2015). Qol is not part of the care of youth with SLE in Egypt despite all the challenges they are facing. This was the motive for conducting the current study.
Multiple scales have been used to evaluate QoL in cSLE (Levy et al., 2014; Putera et al., 2020). Regarding generic questionnaires, Varni et al;(2001) reported that PedsQL™ 4.0 summary score in SLE was significantly lower than healthy children and that PedsQL™ 4.0 can differentiate between healthy children and those with rheumatology disorder. Brunner et al; (2009) reported that CHQ-P50 and the PedsQL™ 4.0 scores of SLE children were lower than healthy children and that the 2 measures are responsive to clinically important changes in cSLE disease activity. In the current study, all domains of the PedsQL™ 4.0 were significantly lower in SLE children compared to published normative data (Varni et al., 2003) and to a previously published data of healthy Egyptian children (Eid et al., 2020) but with no difference in affection between domains which is also inconsistent with Rogers et al. who reported school domain to be the most affected (Rogers et al., 2017) and also a report from USA (Stevens et al., 2019). Noticeably, all domains of PedsQL™ 4.0 of our patients are lower than scores of SLE children reported by Brunner et al., (2009), Stevens et al; (2019) but not different from earlier reports as Varni et al; (2002) (except for social domain). The inconsistency of the results of the studies may be due to differences in selection criteria of patients, ethnic differences which modify disease presentation and severity, differences in patients’ care between countries and the improvement of patients’ care over years.
Multiple rheumatology specific QoL questionnaires are available and some of them have been used for SLE children (Duffy, 2005; Duffy et al., 1997; Wright et al., 1994) with PedsQL™ 3.0 RM introduced by Varni et al; (2002) being the most popular. In the current work, despite having a total PedsQL™ 3.0 RM score above 70 which is recognized to be good QoL score as stated by Varni et al; (2001), the score is still significantly lower than normative data of children with JIA (Brunner et al., 2004) with the worry domain scores being the lowest in our cohort. Comparing our results to the report by Stevens et al; (2019), total and all domain scores of PedsQL™ 3.0 RM were significantly lower in our cohort. Putera et al; (2020) reported PedsQL™ 3.0 RM scores to be much lower in children with LN during induction phase compared to maintenance phase. This again emphasis that that characteristics of patients at time of enrolment in the study have great effect on the scores of HRQOL questionnaires and could account for the inconsistency of results between studies despite using same scales.
In pediatric rheumatology practice, outcome measures are primarily concentrated on the impact of physical disability pain on different aspects of life, as most of the children have JIA. QoL measures that have been developed for children with chiefly musculoskeletal involvement may not adequately assess all aspects of illness in cSLE. Although physical function is an important domain affected in SLE, a true measure of SLE-related QoL must take into consideration other domains of HRQOL and adequately assess the impact of renal and CNS involvement, as well as the effect of medications (Moorthy et al., 2005). So, SLE-specific QoL tool titled (SMILEY©) was developed by Moorthy et al; (2006) and have been validated in different languages including Arabic (Moorthy et al., 2006, 2007). Being a disease-specific scale, SMILEY has no normative data, so we matched our results to previously published SMILEY results from different countries as presented in Table 3 which showed variable results in the different domains. The comparatively high HRQOL among Asian patients could be due to cultural factors, family support, provider preferences related to medication choice that led to disease control or differences in health care systems (Moorthy et al., 2017). Characteristics of patients at time of enrolment in study is also a critical determinant of the questionnaires’ scores. Moorthy et al. (2017) reported the median SLEDAI and SDI scores significantly lower than our cohort. Also, the Brazilian cohort (Moorthy et al., 2013) had a duration of illness 1–153 months and median SLEDAI and SDI scores of 2 and zero respectively which are lower than the current study.
Childhood SLE is more frequent in females which is also spotted in our study and consistent with previous reports from our country and others (Brunner et al., 2009; Eid et al., 2021; Ruperto et al., 2004). Moorthy et al. reported female gender to be associated with lower QoL scores (Moorthy et al., 2017), which was not reported in our study. Patients with LN showed lower scores for SMILEY scale compared to those without LN, but similar finding was not reported in the other 2 scales used. This supports the assumption that SMILEY being disease specific has better ability to evaluate QoL of children with SLE. The relationship of HRQL with renal manifestations, which are generally associated with a worse clinical course and prognosis (Petty & Cassidy, 2001), may reflect a tendency for patients with a progressive disease to report lower HRQL, even if presented with nonthreatening manifestations. The poorer HRQOL in patients with renal and CNS involvement could be related to the aggressive immunosuppressive regimens and higher doses of steroids which change body appearance and lead to impairment of self-confidence especially in adolescents (Ruperto et al., 2004). Patients who received cyclophosphamide had significantly lower SMILEY score than those who didn’t receive it. These findings are coherent with report of Moorthy et al. of 456 children with SLE (Moorthy et al., 2017). Arthritis which is a common feature in SLE was not associated with lower scores of the 3 scales used in the current study. This is against Stevens et al. (2019) who reported arthritis to be the feature affecting all QoL domains. Synovitis and arthritis are typical features of most pediatric rheumatic disorders, but the localization and the extent of the joints inflammation and erosions differs. Arthritis in SLE has short duration, good response to treatment, erosive arthritis is unusual and only a minority of patients develop deformities which was not reported in any of our patients.
Lower disease activity and damage were associated with higher QoL scores of the 3 scales (except for daily activities domain of PedsQL™ 4.0). These findings are consistent with most of published studies (Brunner et al., 2009; Moorthy et al., 2017; Ruperto et al., 2004) except Jones et al. (2013) who reported no such correlation that was explained that traditional measures of cSLE activity do not capture HRQOL outcomes adequately which may limit achievement of optimal health outcomes with cSLE. However, some features and complications of the disease that may also occur in many other chronic illnesses correlated significantly with QoL scores of all 3 scales as long duration of illness, high CGCS, obesity and decreased BMD. The main characteristics and findings of available studies on HRQOL in children with SLE are summarized in Supplementary Table 1.
This study explores for the first time HRQOL in Egyptian children with SLE which was found to be low. Our next step will be the evaluating parents view of their children life quality using the parents’ versions of the questionnaires used in the study which will add to the overall understanding of cSLE Qol in Egyptian youth. Next, Qol assessment will be implemented as part of the routine care and follow up of those children with a psychiatric/ social worker added to our pediatric rheumatology/nephrology care team for SLE children. Focus should be towards controlling disease activity using lowest doses and durations of medications, close monitoring of organ involvements and life quality scores.
Limitations of the current work is being a single center study, the lack of reference normative Egyptian data for the PedsQL™ 3.0 RM and the need for follow up evaluation of HRQOL scores changes over longer follow up duration and the evaluation of family impact scores.
Conclusion
Quality of life assessment in Egyptian children with SLE is not yet included as a part of the standard care provided to these children despite being low as evaluated in the current work. Both generic and disease- or system-specific scales can be used for its assessment. Collaborative efforts from pediatrician, rheumatologists, and psychiatrics are urgently required to support those children.
Data Availability
Data and material are available upon request.
Code Availability
Non applicable.
Abbreviations
- ACR :
-
American college of rheumatology
- BMD :
-
Bone mineral density
- BMI :
-
Body mass index
- CGCS :
-
Cumulative glucocorticoids
- cSLE :
-
Childhood systemic lupus erythematosus
- CNS :
-
Central nervous system.
- eGFR :
-
Estimated Glomerular filtration rate
- HRQOL :
-
Health related quality of life
- JIA :
-
Juvenile idiopathic arthritis
- LN :
-
Lupus nephritis
- MMF :
-
Mycophenolate mofetil
- QoL :
-
Quality of life
- PedsQL ™ 4.0 GCS :
-
Pediatric Quality of Life Inventory Generic Core Scales
- PedsQL3-RM :
-
PedsQL™ 3.0 Rheumatology Module
- SLE :
-
Systemic lupus erythematosus
- SLEDAI :
-
Systemic lupus erythematosus disease activity index
- SDI :
-
SLE International Collaborating Clinics/ American College of Rheumatology Damage Index
- SMILEY :
-
Simple Measure of the Impact of Lupus Erythematosus in Youngsters
References
Brunner, H. I., & Giannini, E. H. (2003). Health-related quality of life in children with rheumatic diseases. Current Opinion in Rheumatology, 15(5), 602–612. https://doi.org/10.1097/00002281-200309000-00014
Brunner, H. I., Klein-Gitelman, M. S., Miller, M. J., Trombley, M., Baldwin, N., Kress, A., Johnson, A. L., Barron, A. C., Griffin, T. A., Passo, M. H., & Lovell, D. J. (2004). Health of children with chronic arthritis: Relationship of different measures and the quality of parent proxy reporting. Arthritis and Rheumatism, 51(5), 763–773. https://doi.org/10.1002/art.20689
Brunner, H. I., Gladman, D. D., Ibañez, D., Urowitz, M. D., & Silverman, E. D. (2008). Difference in disease features between childhood-onset and adult-onset systemic lupus erythematosus. Arthritis and Rheumatism, 58(2), 556–562. https://doi.org/10.1002/art.23204
Brunner, H. I., Higgins, G. C., Wiers, K., Lapidus, S. K., Olson, J. C., Onel, K., Punaro, M., Ying, J., Klein-Gitelman, M. S., & Seid, M. (2009). Health-related quality of life and its relationship to patient disease course in childhood-onset systemic lupus erythematosus. The Journal of Rheumatology, 36(7), 1536–1545. https://doi.org/10.3899/jrheum.081164
Duffy, C. M. (2005). Measurement of health status, functional status, and quality of life in children with juvenile idiopathic arthritis: Clinical science for the pediatrician. Pediatric Clinics of North America, 52(2), 359–v. https://doi.org/10.1016/j.pcl.2005.01.009
Duffy, C. M., Arsenault, L., Duffy, K. N., Paquin, J. D., & Strawczynski, H. (1997). The juvenile arthritis quality of life questionnaire–development of a new responsive index for juvenile rheumatoid arthritis and juvenile spondyloarthritides. The Journal of Rheumatology, 24(4), 738–746.
Eesa, N. N., Abdel Nabi, H., Owaidy, R. E., Khalifa, I., Radwan, A. R., NourEl-Din, A. M., Amer, M. A., ElShereef, R. R., Hassan, E., Ismail, F., El-Gazzar, I. I., Khalil, N. M., Moshrif, A. H., Abualfadl, E., Tharwat, S., Fathi, H. M., Abd Elazeem, M. I., El-Shebini, E., Samy, N., … Egyptian College of Rheumatology (ECR) SLE Study Group. (2021). Systemic lupus erythematosus children in Egypt: Homeland spectrum amid the global situation. Lupus, 30(13), 2135–2143. https://doi.org/10.1177/09612033211043010
Eid, R., Fathy, A. A., & Hamdy, N. (2020). Health-related quality of life in Egyptian children with nephrotic syndrome. Quality of Life Research, 29(8), 2185–2196. https://doi.org/10.1007/s11136-020-02438-0
Eid, R., Hammad, A., Abdelsalam, M., Fathy, A. A., Abd-El Ghafaar, D. M., Elmarghany, E. B., El-Hanafy, A. A., Mostafa, N., Niazey, N. A., Korkor, M. S., & Hamdy, N. (2021). Tumor necrosis factor receptor II and PTPN22 genes polymorphisms and the risk of systemic lupus erythematosus in Egyptian children. Lupus, 30(9), 1449–1458. https://doi.org/10.1177/09612033211020359
Elmougy, A., Sarhan, A., Hammad, A., El-Refaey, A., Zedan, M., Eid, R., Laimon, W., Elrahman, A. A., Elhussieni, F., El-Sherbeny, E., & Bakr, A. (2015). Erratum to: Lupus nephritis in Egyptian children: A 16-year experience. Journal of Nephrology, 28(1), 131. https://doi.org/10.1007/s40620-014-0170-0
El-Ziny, M. A., Al-Tonbary, Y. A., Salama, O. S., Bakr, A., Al-Marsafawy, H., & Elsharkawy, A. A. (2007). Low bone mass in children with malignant lymphoma. Pediatric Hematology and Oncology, 24(8), 577–585. https://doi.org/10.1080/08880010701640275
Gladman, D., Ginzler, E., Goldsmith, C., Fortin, P., Liang, M., Urowitz, M., Bacon, P., Bombardieri, S., Hanly, J., Hay, E., Isenberg, D., Jones, J., Kalunian, K., Maddison, P., Nived, O., Petri, M., Richter, M., Sanchez-Guerrero, J., Snaith, M., … Zoma, A. (1996). The development and initial validation of the Systemic Lupus International Collaborating Clinics/American College of Rheumatology damage index for systemic lupus erythematosus. Arthritis and Rheumatism, 39(3), 363–369. https://doi.org/10.1002/art.1780390303
Gladman, D. D., Ibañez, D., & Urowitz, M. B. (2002). Systemic lupus erythematosus disease activity index 2000. The Journal of Rheumatology, 29(2), 288–291.
Hersh, A. O., von Scheven, E., Yazdany, J., Panopalis, P., Trupin, L., Julian, L., Hersh, A. O., von Scheven, E., Yazdany, J., Panopalis, P., Trupin, L., Julian, L., Katz, P., Criswell, L. A., & Yelin, E. (2009). Differences in long-term disease activity and treatment of adult patients with childhood- and adult-onset systemic lupus erythematosus. Arthritis and Rheumatism, 61(1), 13–20. https://doi.org/10.1002/art.24091
Hochberg, M. C. (1997). Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis and Rheumatism, 40(9), 1725. https://doi.org/10.1002/art.1780400928
Houghton, K. M., Tucker, L. B., Potts, J. E., & McKenzie, D. C. (2008). Fitness, fatigue, disease activity, and quality of life in pediatric lupus. Arthritis and Rheumatism, 59(4), 537–545. https://doi.org/10.1002/art.23534
Jones, J. T., Nelson, S. L., Wootton, J., Liberio, B., Greenler, A. J., Huggins, J. L., Huggins, J. L., Schanberg, L. E., & Brunner, H. (2013). Level and Determinants of Health-Related Quality of Life in Childhood-Onset Systemic Lupus Erythematosus, [Abstract 844]: 2013 ACR/ARHP Annual Meeting.
Levy, D. M., Peschken, C. A., Tucker, L. B., Chédeville, G., Huber, A. M., Pope, J. E., Canadian Network for Improved Outcomes in SLE (CaNIOS) 1000 Faces Investigators, & Silverman, E. D. (2014). Association of health-related quality of life in childhood-onset systemic lupus erythematosus with ethnicity: results from a multiethnic multicenter Canadian cohort. Arthritis Care & Research, 66(12), 1767–1774. https://doi.org/10.1002/acr.22363
Massias, J. S., Smith, E. M., Al-Abadi, E., Armon, K., Bailey, K., Ciurtin, C., Davidson, J., Gardner-Medwin, J., Haslam, K., Hawley, D. P., Leahy, A., Leone, V., McErlane, F., Mewar, D., Modgil, G., Moots, R., Pilkington, C., Ramanan, A. V., Rangaraj, S., … Hedrich, C. M. (2021). Clinical and laboratory phenotypes in juvenile-onset systemic lupus erythematosus across ethnicities in the UK. Lupus, 30(4), 597–607. https://doi.org/10.1177/0961203320984251
Miller, M. L., LeBovidge, J., & Feldman, B. (2002). Health-related quality of life in children with arthritis. Rheumatic Diseases Clinics of North America, 28(3), 493–vi. https://doi.org/10.1016/s0889-857x(02)00019-4
Mina, R., & Brunner, H. I. (2010). Pediatric lupus–are there differences in presentation, genetics, response to therapy, and damage accrual compared with adult lupus? Rheumatic Diseases Clinics of North America, 36(1), 53–viii. https://doi.org/10.1016/j.rdc.2009.12.012
Moorthy, L. N., Peterson, M., Onel, K. B., Harrison, M. J., & Lehman, T. J. (2005). Quality of life in children with systemic lupus erythematosus. Current Rheumatology Reports, 7(6), 447–452. https://doi.org/10.1007/s11926-005-0049-0
Moorthy, L. N., Peterson, M. G., Baratelli, M., Harrison, M. J., Onel, K. B., Chalom, E. C., Haines, K., Hashkes, P. J., & Lehman, T. J. (2006). Simple Measure of The Impact of Lupus Erythematosus In Youngsters (SMILEY): Validity, internal consistency and test-retest reliability. Arthritis and Rheumatism, 54(9), S162.
Moorthy, L. N., Peterson, M. G., Baratelli, M., Harrison, M. J., Onel, K. B., Chalom, E. C., Haines, K., Hashkes, P. J., & Lehman, T. J. (2007). Multicenter validation of a new quality of life measure in pediatric lupus. Arthritis and Rheumatism, 57(7), 1165–1173. https://doi.org/10.1002/art.22988
Moorthy, L. N., Peterson, M. G., Hassett, A. L., Baratelli, M., Chalom, E. C., Hashkes, P. J., Hong, S., Reiff, A., & Lehman, T. J. (2009). Relationship between health-related quality of life and SLE activity and damage in children over time. Lupus, 18(7), 622–629. https://doi.org/10.1177/0961203308101718
Moorthy, L. N., Saad-Magalhães, C., Sato, J. O., Len, C. A., Vasco, M. B., Appenzeller, S., Marini, R., Oliveira, S. K., Rodrigues, M., Sztajnbok, F., Almeida, R. G., Jesus, A. A., Campos, L. M., Silva, C. A., Peterson, M. G., Hassett, A. L., Weiss, E., Verma, S., Dahodwala, M. Q., & Lehman, T. J. (2013). Validation of the Portuguese Simple Measure of Impact of Lupus Erythematosus in Youngsters (SMILEY) in Brazil. Lupus, 22(2), 190–197. https://doi.org/10.1177/0961203312470185
Moorthy, L. N., Baldino, M. E., Kurra, V., Puwar, D., Llanos, A., Peterson, M. G., Hassett, A. L., Lehman, T. J., International SMILEY© Collaborative group. (2017). Relationship between health-related quality of life, disease activity and disease damage in a prospective international multicenter cohort of childhood onset systemic lupus erythematosus patients. Lupus, 26(3), 255–265. https://doi.org/10.1177/0961203316659546
Petty, R. E., & Cassidy, J. T. (2001). Systemic lupus erythematosus. In J. T. Cassidy & R. E. Petty (Eds.), Textbook of pediatric rheumatology (4th ed., pp. 396–449). WB Saunders.
Putera, A. M., Irwanto, I., Maramis, M. M., Prasetyo, R. V., Soemyarso, N. A., & Noer, M. S. (2020). Effect of mental health problems on the quality of life in children with lupus nephritis. Neuropsychiatric Disease and Treatment, 16, 1583–1593. https://doi.org/10.2147/NDT.S250373
Ravelli, A., Ruperto, N., & Martini, A. (2005). Outcome in juvenile onset systemic lupus erythematosus. Current Opinion in Rheumatology, 17(5), 568–573. https://doi.org/10.1097/01.bor.0000169364.69066.1e
Rogers, J., Sagcal-Gironella, A. C. P., Rosillo, P., Ramirez, A. A., Banuelos, R., & de Guzman, M. M. (2017). A single center review of health-related quality of life in children with systemic lupus erythematosus using the pediatric quality of life inventory version 4.0 generic core scale. Arthritis & Rheumatology, 69(4), 223–224.
Ruperto, N., Buratti, S., Duarte-Salazar, C., Pistorio, A., Reiff, A., Bernstein, B., Maldonado-Velázquez, M. R., Beristain-Manterola, R., Maeno, N., Takei, S., Falcini, F., Lepore, L., Spencer, C. H., Pratsidou-Gertsi, P., Martini, A., & Ravelli, A. (2004). Health-related quality of life in juvenile-onset systemic lupus erythematosus and its relationship to disease activity and damage. Arthritis and Rheumatism, 51(3), 458–464. https://doi.org/10.1002/art.20412
Stevens, B., Rodriguez, M., Rakestraw, A., & O’neil, K. (2019). SAT0477 quality of life in subjects with pre-pubertal onset systemic lupus erythematosus. Annals of the Rheumatic, 78, 1326–1327.
Thumboo, J., & Strand, V. (2007). Health-related quality of life in patients with systemic lupus erythematosus: An update. Annals of the Academy of Medicine, Singapore, 36(2), 115–122.
Varni, J. W., Burwinkle, T. M., Seid, M., & Skarr, D. (2003). The PedsQL 4.0 as a pediatric population health measure: feasibility, reliability, and validity. Ambulatory Pediatrics: the Official Journal of the Ambulatory Pediatric Association, 3(6), 329–341. https://doi.org/10.1367/1539-4409(2003)003%3c0329:tpaapp%3e2.0.co;2
Varni, J. W., Seid, M., & Kurtin, P. S. (2001). PedsQL 4.0: reliability and validity of the pediatric quality of life inventory version 4.0 generic core scales in healthy and patient populations. Medical Care, 39(8), 800–812. https://doi.org/10.1097/00005650-200108000-00006
Varni, J. W., Seid, M., Smith Knight, T., Burwinkle, T., Brown, J., & Szer, I. S. (2002). The PedsQL in pediatric rheumatology: Reliability, validity, and responsiveness of the pediatric quality of life inventory generic core scales and rheumatology module. Arthritis and Rheumatism, 46(3), 714–725. https://doi.org/10.1002/art.10095
Wright, F. V., Law, M., Crombie, V., Goldsmith, C. H., & Dent, P. (1994). Development of a self-report functional status index for juvenile rheumatoid arthritis. The Journal of Rheumatology, 21(3), 536–544.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). This research received no funding.
Author information
Authors and Affiliations
Contributions
RE: research hypothesis, obtained questionnaires’ permission to use, statistical analysis, wrote initial draft, revised, and approved the final manuscript. AH, NH: research protocol, revised and approved the final manuscript, provided medical care to all patients. MSK, SR: research protocol, guided the questionnaires fulfilment by patients, revised final manuscript, cardiac assessment of all patients, revised and approved the final manuscript. AAF, DMAE: research protocol, guided the questionnaires fulfilment by patients, statistical analysis, and revised and approved final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declared no potential conflicts of interest concerning the research, authorship, and/or publication of this article.
Ethical Approval
All procedures performed in study were in accordance with the ethical standards of the institutional and/or national research committee and with the 1975 Helsinki declaration and its later amendments or comparable ethical standards. This study was reviewed and approved by the local ethical committee (MFM-IRB) (Code: R.21.08.1393).
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Consent for Publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Eid, R., Hammad, A., Korkor, M.S. et al. Health Related Quality of Life in Juvenile-Onset Systemic Lupus Erythematosus: A Questionnaire-Based Study. Matern Child Health J 27, 1578–1588 (2023). https://doi.org/10.1007/s10995-023-03680-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10995-023-03680-x