Abstract
Context
Global climate change poses a significant threat to the habitat connectivity of cold-water-adapted organisms, leading to species extinctions. If gene flow can be modeled by landscape variables, changes in connectivity among populations could be predicted. However, in dendritic and heterogeneous stream ecosystems, few studies have estimated the changes in gene flow from genetic data, in part due to the difficulty in applying landscape genetics methods and accessing water temperature information.
Objectives
Inferring the determinants and future changes of the gene flow in the cold-water adapted fluvial sculpin Cottus nozawae using a recently developed model-based riverscape genetics technique and a hydrological model for estimating water temperature.
Methods
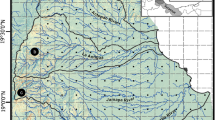
The strength of gene flow on each stream section was modeled by watershed-wide riverscape variables and genome-wide SNP data for C. nozawae in the upper reaches of the Sorachi River, Hokkaido, Japan. Future changes in gene flow were inferred by this model and hydrologically estimated water temperatures under the high greenhouse gas concentration scenario (IPCC RCP8.5).
Results
Stream order, water temperature, slope, and distance were selected as riverscape variables affecting the strength of gene flow in each stream section. In particular, the trend of greater gene flow in sections with higher stream order and lower temperature fluctuations or summer water temperatures was pronounced. The map from the model showed that gene flow is overall prevented in small tributaries in the southern area, where spring-fed environments are less prevalent. Estimating future changes, gene flow was predicted to decrease dramatically at the end of the twenty-first century.
Conclusions
Our results demonstrated that the connectivity of cold-water sculpin populations is expected to decline dramatically in a changing climate. Riverscape genetic modeling is useful for gaining information on population connectivity that does not fully coincide with habitat suitability.



Similar content being viewed by others
Data availability
Genetic and environmental data generated in this study were deposited at Figshare (https://doi.org/10.6084/m9.figshare.19694989).
References
Almodóvar A, Nicola GG, Ayllón D, Elvira B (2012) Global warming threatens the persistence of Mediterranean brown trout. Glob Chang Biol 18:1549–1560
Aunins AW, Petty JT, King TL et al (2015) River mainstem thermal regimes influence population structuring within an appalachian brook trout population. Conserv Genet 16:15–29
Balkenhol N, Cushman SA, Storfer A, Waits LP (2015) Introduction to landscape genetics—concepts, methods, applications. In: Balkenhol N, Cushman SA, Storfer A, Waits LP (eds) Landscape Genetics. Wiley, New York, pp 1–8
Barbarossa V, Bosmans J, Wanders N et al (2021) Threats of global warming to the world’s freshwater fishes. Nat Commun 12:1701
Bowcock AM, Ruiz-Linares A, Tomfohrde J et al (1994) High resolution of human evolutionary trees with polymorphic microsatellites. Nature 368:455–457
Cain MK, Zhang Z (2019) Fit for a Bayesian: An evaluation of PPP and DIC for structural equation modeling. Struct Equ Model 26:39–50
Caldera EJ, Bolnick DI (2008) Effects of colonization history and landscape structure on genetic variation within and among threespine stickleback (Gasterosteus aculeatus) populations in a single watershed. Evol Ecol Res 10:575–598
Campbell Grant EH, Lowe WH, Fagan WF (2007) Living in the branches: population dynamics and ecological processes in dendritic networks. Ecol Lett 10:165–175
Catchen J, Hohenlohe PA, Bassham S et al (2013) Stacks: an analysis tool set for population genomics. Mol Ecol 22:3124–3140
Chafin TK, Mussmann SM, Douglas MR, Douglas ME (2021) Quantifying isolation-by-resistance and connectivity in dendritic ecological networks. bioRxiv. https://doi.org/10.1101/2021.03.25.437078
Comte L, Buisson L, Daufresne M, Grenouillet G (2012) Climate-induced changes in the distribution of freshwater fish: observed and predicted trends. Freshw Biol 58:625–639
Davis CD, Epps CW, Flitcroft RL, Banks MA (2018) Refining and defining riverscape genetics: how rivers influence population genetic structure. Wires Water 5:e1269
dos Oliveira JA, Farias IP, Costa GC, Werneck FP (2019) Model-based riverscape genetics: disentangling the roles of local and connectivity factors in shaping spatial genetic patterns of two Amazonian turtles with different dispersal abilities. Evol Ecol 33:273–298
Earl DA, vonHoldt BM (2012) STRUCTURE HARVESTER: a website and program for visualizing structure output and implementing the Evanno method. Conserv Genet Resour 4:359–361
Elith J, Leathwick JR (2009) Species distribution models: ecological explanation and prediction across space and time. Annu Rev Ecol Evol Syst 40:677–697
Escalante MA, García-De León FJ, Ruiz-Luna A et al (2018) The interplay of riverscape features and exotic introgression on the genetic structure of the Mexican golden trout (Oncorhynchus chrysogaster), a simulation approach. J Biogeogr 45:1500–1514
Escalante MA, Perrier C, García-De León FJ et al (2020) Genotyping-by-sequencing reveals the effects of riverscape, climate and interspecific introgression on the genetic diversity and local adaptation of the endangered Mexican golden trout (Oncorhynchus chrysogaster). Conserv Genet 21:907–926
Evanno G, Regnaut S, Goudet J (2005) Detecting the number of clusters of individuals using the software STRUCTURE: A simulation study. Mol Ecol 14:2611–2620
García Molinos J, Ishiyama N, Sueyoshi M, Nakamura F (2022) Timescale mediates the effects of environmental controls on water temperature in mid- to low-order streams. Sci Rep 12:12248
Goudet J (1995) FSTAT (Version 1.2): a computer program to calculate F-statistics. J Hered 86:485–486
Grummer JA, Beheregaray LB, Bernatchez L (2019) Aquatic landscape genomics and environmental effects on genetic variation. Trends Ecol Evol 34:641–654
Han CC, Tew KS, Fang LS (2007) Spatial and temporal variations of two cyprinids in a subtropical mountain reserve—a result of habitat disturbance. Ecol Freshw Fish 16:395–403
Hand BK, Muhlfeld CC, Wade AA et al (2016) Climate variables explain neutral and adaptive variation within salmonid metapopulations: the importance of replication in landscape genetics. Mol Ecol 25:689–705
Hijmans RJ, Cameron SE, Parra JL et al (2005) Very high resolution interpolated climate surfaces for global land areas. Int J Climatol 25:1965–1978
Holsinger KE, Weir BS (2009) Genetics in geographically structured populations: defining, estimating and interpreting FST. Nat Rev Genet 10:639–650
Hubisz MJ, Falush D, Stephens M, Pritchard JK (2009) Inferring weak population structure with the assistance of sample group information. Mol Ecol Resour 9:1322–1332
Inoue K, Berg DJ (2017) Predicting the effects of climate change on population connectivity and genetic diversity of an imperiled freshwater mussel, Cumberlandia monodonta (Bivalvia: Margaritiferidae), in riverine systems. Glob Chang Biol 23:94–107
IPCC (2014) Summary for policymakers. In: Field CB, Barros VR, Dokken DJ et al (eds) Climate change 2014: impacts, adaptation, and vulnerability. Cambridge University Press, Cambridge and New York, pp 1–32. https://www.ipcc.ch/report/ar5/wg2/
Ishiyama N, Sueyoshi M, García Molinos J et al (2023) Underlying geology and climate interactively shape climate change refugia in mountain streams. Ecol Monogr. https://doi.org/10.1002/ecm.1566
Kanno Y, Vokoun JC, Letcher BH (2011) Fine-scale population structure and riverscape genetics of brook trout (Salvelinus fontinalis) distributed continuously along headwater channel networks. Mol Ecol 20:3711–3729
Koizumi I, Maekawa K (2004) Metapopulation structure of stream-dwelling Dolly Varden charr inferred from patterns of occurrence in the Sorachi River basin, Hokkaido, Japan. Freshw Biol 49:973–981
Koizumi I, Kanazawa Y, Tanaka Y (2013) The fishermen were right: experimental evidence for tributary refuge hypothesis during floods. Zool Sci 30:375–379
Kopelman NM, Mayzel J, Jakobsson M et al (2015) Clumpak: a program for identifying clustering modes and packaging population structure inferences across K. Mol Ecol Resour 15:1179–1191
Kottler EJ, Dickman EE, Sexton JP et al (2021) Draining the swamping hypothesis: little evidence that gene flow reduces fitness at range edges. Trends Ecol Evol 36:533–544
Lamphere BA, Blum MJ (2012) Genetic estimates of population structure and dispersal in a benthic stream fish. Ecol Freshw Fish 21:75–86
Landguth EL, Cushman SA, Schwartz MK et al (2010) Quantifying the lag time to detect barriers in landscape genetics. Mol Ecol 19:4179–4191
Landguth EL, Bearlin A, Day CC, Dunham J (2016) CDMetaPOP: an individual-based, eco-evolutionary model for spatially explicit simulation of landscape demogenetics. Methods Ecol Evol 8:4–11
Leroy G, Carroll EL, Bruford MW et al (2018) Next-generation metrics for monitoring genetic erosion within populations of conservation concern. Evol Appl 11:1066–1083
Manel A, Holdergger R (2013) Ten years of landscape genetics. Trends Ecol Evol 28:614–621
Mateo-Sánchez MC, Balkenhol N, Cushman S et al (2015) A comparative framework to infer landscape effects on population genetic structure: are habitat suitability models effective in explaining gene flow? Landsc Ecol 30:1405–1420
McRae BH (2006) Isolation by resistance. Evolution 60:1551–1561
McRae BH, Beier P (2007) Circuit theory predicts gene flow in plant and animal populations. Proc Natl Acad Sci U S A 104:19885–19890
Nagasaka A, Sugiyama S (2010) Factors affecting the summer maximum stream temperature of small streams in northern Japan. Bull Hokkaido for Res Inst 47:35–43 (In Japanese with English abstract)
Nakajima S, Sueyoshi M, Hirota SK et al (2021) A strategic sampling design revealed the local genetic structure of cold-water fluvial sculpin: a focus on groundwater-dependent water temperature heterogeneity. Heredity 127:413–422
Nakamura F (2022) Riparian forests and climate change: interactive zone of green and blue infrastructure. In: Nakamura F (ed) Green infrastructure and climate change adaptation. Springer, Singapore, pp 73–91
Nei M (1973) Analysis of gene diversity in subdivided populations. Proc Natl Acad Sci U S A 70:3321–3323
Oksanen JF, Blanchet G, Friendly M et al (2019) vegan: community ecology package. R package version 2.5–6. https://CRAN.R-project.org/package=vegan
Paris JR, Stevens JR, Catchen JM (2017) Lost in parameter space: a road map for stacks. Methods Ecol Evol 8:1360–1373
Paz-Vinas I, Blanchet S (2015) Dendritic connectivity shapes spatial patterns of genetic diversity: a simulation-based study. J Evol Biol 28:986–994
Peakall R, Smouse PE (2012) GenAlEx 6.5: Genetic analysis in Excel. Population genetic software for teaching and research-an update. Bioinformatics 28:2537–2539
Peterson EE, Hanks EM, Hooten MB et al (2019) Spatially structured statistical network models for landscape genetics. Ecol Monogr 89:e01355
Pritchard JK, Stephens M, Donnelly P (2000) Inference of population structure using multilocus genotype data. Genetics 155:945–959
R Core Team (2019) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Rahel FJ, Keleher CJ, Anderson JL (1996) Potential habitat loss and population fragmentation for cold water fish in the North Platte River drainage of the Rocky Mountains: Response to climate warming. Limnol Oceanogr 41:1116–1123
Sartor CC, Wan HY, Pereira JA et al (2022) Landscape genetics outperforms habitat suitability in predicting landscape resistance for congeneric cat species. J Biogeogr 49:2206–2217
Saura S, Pascual-Hortal L (2007) A new habitat availability index to integrate connectivity in landscape conservation planning: comparison with existing indices and application to a case study. Landsc Urban Plan 83:91–103
Savary P, Foltête JC, Moal H et al (2021) graph4lg: A package for constructing and analysing graphs for landscape genetics in R. Methods Ecol Evol 12:539–547
Spear SF, Cushman SA, McRae BH (2015) Resistance surface modeling in landscape genetics. In: Balkenhol N, Cushman SA, Storfer A, Waits LP (eds) Landscape genetics. Wiley, New York, pp 129–148
Sugawara M (1979) Automatic calibration of the tank model. Hydrol Sci Bull 24:375–388
Suyama Y, Matsuki Y (2015) MIG-seq: An effective PCR-based method for genome-wide single-nucleotide polymorphism genotyping using the next-generation sequencing platform. Sci Rep 5:16963
Suyama Y, Hirota SK, Matsuo A et al (2022) Complementary combination of multiplex high-throughput DNA sequencing for molecular phylogeny. Ecol Res 37:171–181
Suzuki K, Ishiyama N, Koizumi I, Nakamura F (2021) Combined effects of summer water temperature and current velocity on the distribution of a cold-water-adapted sculpin (Cottus nozawae). Water 13:975
Suzuki H, Nakatsugawa M, Ishiyama N (2022) Climate change impacts on stream water temperatures in the snowy cold region according to geological conditions. Water 14:2166
Ueda S, Nakatsugawa M, Usutani T (2020) Estimation of high-resolution downscaled climate information based on verification of water balance in watershed of Hokkaido. J Jpn Soc Civil Eng. https://doi.org/10.2208/jscejhe.76.2_I_25 (In Japanese with English Abstract)
Wasserman TN, Cushman SA, Schwartz MK, Wallin DO (2010) Spatial scaling and multi-model inference in landscape genetics: Martes americana in northern Idaho. Landsc Ecol 25:1601–1612
Wasserman TN, Cushman SA, Shirk AS et al (2012) Simulating the effects of climate change on population connectivity of American marten (Martes americana) in the northern Rocky Mountains, USA. Landsc Ecol 27:211–225
White SL, Hanks EM, Wagner T (2020) A novel quantitative framework for riverscape genetics. Ecol Appl 30:e02147
Woodward G, Perkins DM, Brown LE (2010) Climate change and freshwater ecosystems: Impacts across multiple levels of organization. Philos Trans R Soc B Biol Sci 365:2093–2106
Wright S (1943) Isolation by distance. Genetics 28:114–138
Yagami T, Goto A (2000) Patchy distribution of a fluvial sculpin, Cottus nozawae, in the Gakko River system at the southern margin of its native range. Ichthyol Res 47:277–286
Zeller KA, McGarigal K, Whiteley AR (2012) Estimating landscape resistance to movement: a review. Landsc Ecol 27:777–797
Acknowledgements
We thank Nobuo Ishiyama for his cooperation in validating the hydrological model. This study is partly supported by the research fund for the Ishikari and Tokachi Rivers provided by the Ministry of Land, Infrastructure, Transport and Tourism of Japan.
Funding
This study is partly supported by the research fund for the Ishikari and Tokachi Rivers provided by the Ministry of Land, Infrastructure, Transport and Tourism of Japan.
Author information
Authors and Affiliations
Contributions
Conceptualization, S.N. and F.N.; Data curation, S.N. and S.K.H.; Formal analysis, S.N.; Funding acquisition; F.N.; Investigation, S.N., H.S., A.M., and S.K.H.; Methodology, S.N., H.S., M.N., and Y.S.; Project administration, F.N.; Resources, S.N., M.N., A.M., and Y.S.; Software, S.N. and H.S., A.M., and S.K.H.; Supervision, M.N., Y.S. and F.N.; Validation, S.N. and F.N.; Visualization, S.N.; Writing – original draft, S.N. and H.S.; Writing – review & editing, S.N., H.S., M.N., A.M., S.K.H., Y.S., and F.N.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nakajima, S., Suzuki, H., Nakatsugawa, M. et al. Inferring future changes in gene flow under climate change in riverscapes: a pilot case study in fluvial sculpin. Landsc Ecol 38, 1351–1362 (2023). https://doi.org/10.1007/s10980-023-01633-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10980-023-01633-x